
HL Paper 2
Nitrogen monoxide reacts at 1280 °C with hydrogen to form nitrogen and water. All reactants and products are in the gaseous phase.
The gas-phase decomposition of dinitrogen monoxide is considered to occur in two steps.
\[\begin{array}{*{20}{l}} {{\text{Step 1:}}}&{{{\text{N}}_2}{\text{O(g)}}\xrightarrow{{{k_1}}}{{\text{N}}_2}({\text{g)}} + {\text{O(g)}}} \\ {{\text{Step 2:}}}&{{{\text{N}}_2}{\text{O(g)}} + {\text{O(g)}}\xrightarrow{{{k_2}}}{{\text{N}}_2}({\text{g)}} + {{\text{O}}_2}{\text{(g)}}} \end{array}\]
The experimental rate expression for this reaction is rate \( = k{\text{[}}{{\text{N}}_2}{\text{O]}}\).
The conversion of \({\text{C}}{{\text{H}}_{\text{3}}}{\text{NC}}\) into \({\text{C}}{{\text{H}}_{\text{3}}}{\text{CN}}\) is an exothermic reaction which can be represented as follows.
\({\text{C}}{{\text{H}}_{\text{3}}}–{\text{N}}\)\( \equiv \)\({\text{C}} \to {\text{transition state}} \to {\text{C}}{{\text{H}}_{\text{3}}}–{\text{C}}\)\( \equiv \)\({\text{N}}\)
This reaction was carried out at different temperatures and a value of the rate constant, \(k\), was obtained for each temperature. A graph of \(\ln k\) against \(1/T\) is shown below.
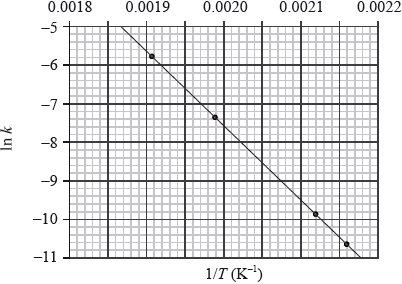
Define the term rate of reaction.
State an equation for the reaction of magnesium carbonate with dilute hydrochloric acid.
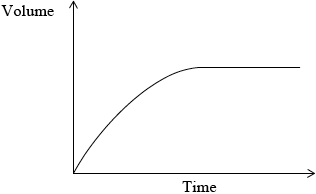
The rate of this reaction in (a) (ii), can be studied by measuring the volume of gas collected over a period of time. Sketch a graph which shows how the volume of gas collected changes with time.
The experiment is repeated using a sample of hydrochloric acid with double the volume, but half the concentration of the original acid. Draw a second line on the graph you sketched in part (a) (iii) to show the results in this experiment. Explain why this line is different from the original line.
The kinetics of the reaction were studied at this temperature. The table shows the initial rate of reaction for different concentrations of each reactant.
Deduce the order of the reaction with respect to NO and \({{\text{H}}_2}\), and explain your reasoning.
Deduce the rate expression for the reaction.
Determine the value of the rate constant for the reaction from Experiment 3 and state its units.
Identify the rate-determining step.
Identify the intermediate involved in the reaction.
Define the term activation energy, \({E_{\text{a}}}\).
Construct the enthalpy level diagram and label the activation energy, \({E_{\text{a}}}\), the enthalpy change, \(\Delta H\), and the position of the transition state.
Describe qualitatively the relationship between the rate constant, \(k\), and the temperature, \(T\).
Calculate the activation energy, \({E_{\text{a}}}\), for the reaction, using Table 1 of the Data Booklet.
Markscheme
decrease in concentration/mass/amount/volume of reactant with time / increase in concentration/mass/amount/volume of product with time / change in concentration/mass/amount/volume of reactant/product with time;
\({\text{MgC}}{{\text{O}}_3}{\text{(s)}} + {\text{2HCl(aq)}} \to {\text{MgC}}{{\text{l}}_2}{\text{(aq)}} + {\text{C}}{{\text{O}}_2}{\text{(g)}} + {{\text{H}}_2}{\text{O(l)}}\);
Ignore state symbols.
 ;
;
Plot starts at the origin and levels off.
No mark awarded if axes are not labelled.
new curve reaches same height as original curve;
new curve less steep than original curve;
volume of gas produced is the same because the same amount of acid is used;
reaction is slower because concentration is decreased;
(from experiments 1 and 2 at constant \({\text{[}}{{\text{H}}_2}{\text{]}}\)), [NO] doubles, rate quadruples;
hence, second order with respect to NO;
(from experiments 2 and 3 at constant [NO]), \({\text{[}}{{\text{H}}_{\text{2}}}{\text{]}}\)doubles, rate doubles;
first order with respect to \({{\text{H}}_2}\);
Allow alternative mathematical deductions also.
\({\text{rate}} = k{{\text{[NO]}}^{\text{2}}}{\text{[}}{{\text{H}}_{\text{2}}}{\text{]}}\);
\(k\left( { = (10.00 \times {{10}^{ - 5}})/{{(10.00 \times {{10}^{ - 3}})}^2}(4.00 \times {{10}^{ - 3}})} \right) = 2.50 \times {10^2}\);
Do not penalize if Experiments 1 or 2 are used to determine k.
\({\text{mo}}{{\text{l}}^{ - 2}}{\text{d}}{{\text{m}}^6}{{\text{s}}^{ - 1}}\);
step 1 / equation showing step 1;
O (atom) / oxygen atom;
Do not allow oxygen or O2.
(minimum) energy needed for a reaction to occur / difference in energy between the reactants and transition state;
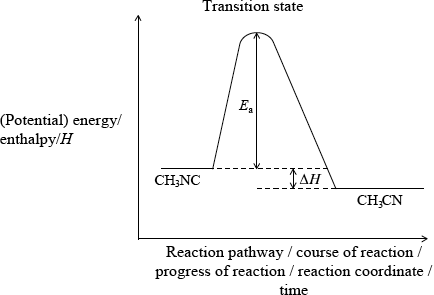
correct position of activation energy;
correct position of \(\Delta H\) and \(H{\text{(C}}{{\text{H}}_3}{\text{NC)}}\)/reactant line above \(H{\text{(C}}{{\text{H}}_3}{\text{CN)}}\) product line;
Accept \(\Delta E\) instead of \(\Delta H\) on diagram if y-axis is labelled as energy.
Do not penalize if CH3NC and CH3CN are not labelled on diagram.
correct position of transition state;
Allow [2 max] if axes are not labelled on diagram.
as temperature/\(T\) increases rate constant/k increases (exponentially);
from graph gradient \(m = - \frac{{{E_{\text{a}}}}}{R}\);
measurement of gradient from chosen points on graph;
Units of m are K. Do not penalize if not given, but do not award mark for incorrect units.
Value of m is based on any two suitable points well separated on the plot.
correct answer for \({E_{\text{a}}}\);
correct units corresponding to answer;
Note: A typical answer for Ea = 1.6 \( \times \) 102 kJ / kJ\(\,\)mol–1.
Examiners report
Surprisingly, the rate of reaction was only correctly defined by approximately 50% of candidates in (a) (i).
The equation for the reaction of magnesium carbonate with dilute hydrochloric acid was not well answered (part (ii)), and often candidates did not write correct formula or forgot to include water as a product.
Part (iii) was well answered by most candidates.
Part (iv) was well answered by most candidates, although the weaker candidates often only scored two or three marks.
Part (b) (i) was well answered and many candidates scored all four marks. Some candidates used a simple mathematical approach and those that followed this method typically were able to deduce the order correctly.
For (ii) most candidates were able to write the rate expression for the reaction.
In (iii), determining the value of the rate constant and its corresponding units was difficult for many candidates and only the better candidates scored both marks. Many mistakes were seen in the units.
Part (c) (i) was usually well answered.
A common mistake for (ii) involved candidates writing \({{\text{O}}_{\text{2}}}\) instead of O.
The definition of activation energy was well answered.
Part (ii) was a question where most candidates scored at least one/two marks although perfect answers were less common. Reasons leading to the loss of marks included: absence of axes, incomplete libelling of axes and the incorrect identification of the position of the transition state.
Parts (iii) and (iv) were very poorly answered for such a fundamental topic. All sorts of errors were evident, including incorrect gradients, inability to rearrange the Arrhenius Equation etc.
Even the better candidates struggled greatly with this question, even though this comes straight from AS 16.3.2.
Calcium carbonate reacts with hydrochloric acid.
CaCO3(s) + 2HCl(aq) → CaCl2(aq) + H2O(l) + CO2(g)
The results of a series of experiments in which the concentration of HCl was varied are shown below.
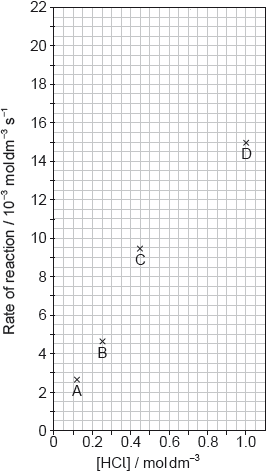
Outline two ways in which the progress of the reaction can be monitored. No practical details are required.
Suggest why point D is so far out of line assuming human error is not the cause.
Draw the best fit line for the reaction excluding point D.
Suggest the relationship that points A, B and C show between the concentration of the acid and the rate of reaction.
Deduce the rate expression for the reaction.
Calculate the rate constant of the reaction, stating its units.
Predict from your line of best fit the rate of reaction when the concentration of HCl is 1.00 mol dm−3.
Describe how the activation energy of this reaction could be determined.
Markscheme
Any two of:
loss of mass «of reaction mixture/CO2»
«increase in» volume of gas produced
change of conductivity
change of pH
change in temperature
Do not accept “disappearance of calcium carbonate”.
Do not accept “gas bubbles”.
Do not accept “colour change” or “indicator”.
[2 marks]
reaction is fast at high concentration AND may be difficult to measure accurately
OR
so many bubbles of CO2 produced that inhibit contact of HCl(aq) with CaCO3(s)
OR
insufficient change in conductivity/pH at high concentrations
OR
calcium carbonate has been used up/is limiting reagent/ there is not enough calcium carbonate «to react with the high concentration of HCl»
OR
HCl is in excess
OR
so many bubbles of CO2 produced that inhibit contact of HCl(aq) with CaCO3(s)
[1 mark]
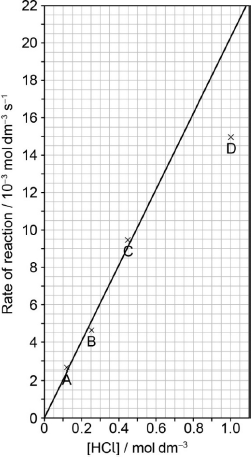
straight line going through the origin AND as close to A, B, C as is reasonably possible
[1 mark]
«directly» proportional
Accept “first order” or “linear”.
Do not accept “rate increases as concentration increases” or “positive correlation”.
[1 mark]
rate = k [H+]
Accept “rate = k [HCl]”.
[1 mark]
0.02
s–1
[2 marks]
20.5 \( \times \) 10–3 «mol dm–3 s–1»
Accept any answer in the range 19.5–21.5.
[1 mark]
ALTERNATIVE 1:
carry out reaction at several temperatures
plot \(\frac{1}{{\text{T}}}\) against log rate constant
Ea = – gradient \( \times \) R
ALTERNATIVE 2:
carry out reaction at two temperatures
determine two rate constants
OR
determine the temperature coefficient of the rate
use the formula \(\ln \frac{{{k_1}}}{{{k_2}}} = \frac{{{E_{\text{a}}}}}{R}\left( {\frac{1}{{{T_2}}} - \frac{1}{{{T_1}}}} \right)\)
Accept “gradient = \(\frac{{ - {E_{\text{a}}}}}{R}\)” for M3.
Award both M2 and M3 for the formula \({\text{ln}}\frac{{rat{e_1}}}{{rat{e_2}}} = \frac{{{E_{\text{a}}}}}{R}\left( {\frac{1}{{{T_2}}} - \frac{1}{{{T_1}}}} \right)\).
Accept any variation of the formula, such as \(\frac{{rat{e_1}}}{{rat{e_2}}} = {e^{ - \frac{{{E_{\text{a}}}}}{R}\left( {\frac{1}{{{T_1}}} - \frac{1}{{{T_2}}}} \right)}}\).
[3 marks]
Examiners report
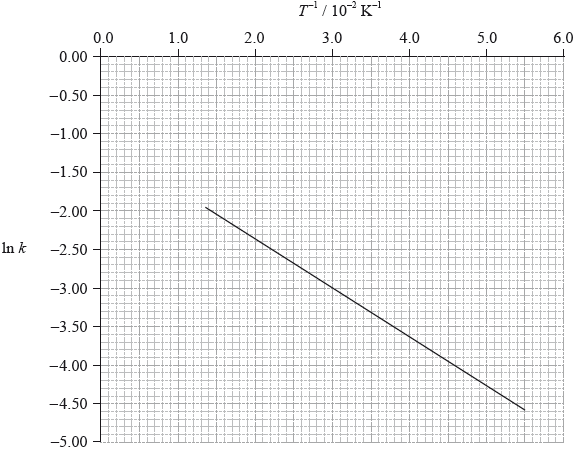
To determine the activation energy of a reaction, the rate of reaction was measured at different temperatures. The rate constant, \(k\), was determined and \(\ln k\) was plotted against the inverse of the temperature in Kelvin, \({T^{ - 1}}\). The following graph was obtained.
Define the term activation energy, \({E_{\text{a}}}\).
Use the graph on page 8 to determine the value of the activation energy, \({E_{\text{a}}}\), in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\).
On the graph on page 8, sketch the line you would expect if a catalyst is added to the reactants.
Markscheme
minimum energy needed to react/start a reaction / energy difference between reactants and transition state;
gradient of the line: –63;
Accept –60 to –65.
\({E_{\text{a}}}{\text{ }}( = - R \times {\text{gradient}}) = 0.52{\text{ (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
Accept 0.50 to 0.54.
gradient of the line less steep (less negative);
Accept any position as long as gradient less steep.
Examiners report
The idea of activation energy being a minimum was seldom communicated. Few were able to follow through all the mathematics to find \({E_{\text{a}}}\) by a graphical method and those that did had often omitted \({\text{1}}{{\text{0}}^{ - 2}}\) in their calculations. The answers were often poorly set out so it was difficult to assess the award of part marks; indeed, many candidates seemed to hope that a correct answer would somehow emerge from a mass of incomprehensible figures.
The idea of activation energy being a minimum was seldom communicated. Few were able to follow through all the mathematics to find \({E_{\text{a}}}\) by a graphical method and those that did had often omitted \({\text{1}}{{\text{0}}^{ - 2}}\) in their calculations. The answers were often poorly set out so it was difficult to assess the award of part marks; indeed, many candidates seemed to hope that a correct answer would somehow emerge from a mass of incomprehensible figures.
The idea of activation energy being a minimum was seldom communicated. Few were able to follow through all the mathematics to find \({E_{\text{a}}}\) by a graphical method and those that did had often omitted \({\text{1}}{{\text{0}}^{ - 2}}\) in their calculations. The answers were often poorly set out so it was difficult to assess the award of part marks; indeed, many candidates seemed to hope that a correct answer would somehow emerge from a mass of incomprehensible figures. The gradient of the graph for (c) was generously marked; all candidates had to do was to realize that the catalyst would lower the activation energy and thus the gradient would be less negative. As long as a line with less negative gradient was drawn, the mark was awarded.
Consider the following reaction studied at 263 K.
\[{\text{2NO(g)}} + {\text{C}}{{\text{l}}_{\text{2}}}{\text{(g)}} \rightleftharpoons {\text{2NOCl(g)}}\]
It was found that the forward reaction is first order with respect to \({\rm{C}}{{\rm{l}}_2}\) and second order with respect to NO. The reverse reaction is second order with respect to NOCl.
Consider the following equilibrium reaction.
\[\begin{array}{*{20}{c}} {{\text{C}}{{\text{l}}_2}({\text{g)}} + {\text{S}}{{\text{O}}_2}({\text{g)}} \rightleftharpoons {\text{S}}{{\text{O}}_2}{\text{C}}{{\text{l}}_2}({\text{g)}}}&{\Delta {H^\Theta } = - 84.5{\text{ kJ}}} \end{array}\]
In a \({\text{1.00 d}}{{\text{m}}^{\text{3}}}\) closed container, at 375 °C, \({\text{8.60}} \times {\text{1}}{{\text{0}}^{ - 3}}{\text{ mol}}\) of \({\text{S}}{{\text{O}}_{\text{2}}}\) and \({\text{8.60}} \times {\text{1}}{{\text{0}}^{ - 3}}{\text{ mol}}\) of \({\text{C}}{{\text{l}}_{\text{2}}}\) were introduced. At equilibrium, \({\text{7.65}} \times {\text{1}}{{\text{0}}^{ - 4}}{\text{ mol}}\) of \({\text{S}}{{\text{O}}_{\text{2}}}{\text{C}}{{\text{l}}_{\text{2}}}\) was formed.
State the rate expression for the forward reaction.
Predict the effect on the rate of the forward reaction and on the rate constant if the concentration of NO is halved.
1.0 mol of \({\rm{C}}{{\rm{l}}_2}\) and 1.0 mol of NO are mixed in a closed container at constant temperature. Sketch a graph to show how the concentration of NO and NOCl change with time until after equilibrium has been reached. Identify the point on the graph where equilibrium is established.
Consider the following reaction.
\[{\text{N}}{{\text{O}}_2}{\text{(g)}} + {\text{CO(g)}} \to {\text{NO(g)}} + {\text{C}}{{\text{O}}_2}{\text{(g)}}\]
Possible reaction mechanisms are:
\(\begin{array}{*{20}{l}} {{\text{Above 775 K:}}}&{{\text{N}}{{\text{O}}_2} + {\text{CO}} \to {\text{NO}} + {\text{C}}{{\text{O}}_{\text{2}}}}&{{\text{slow}}} \\ {{\text{Below 775 K:}}}&{{\text{2N}}{{\text{O}}_2} \to {\text{NO}} + {\text{N}}{{\text{O}}_{\text{3}}}}&{{\text{slow}}} \\ {}&{{\text{N}}{{\text{O}}_3} + {\text{CO}} \to {\text{N}}{{\text{O}}_2} + {\text{C}}{{\text{O}}_2}}&{{\text{fast}}} \end{array}\)
Based on the mechanisms, deduce the rate expressions above and below 775 K.
State two situations when the rate of a chemical reaction is equal to the rate constant.
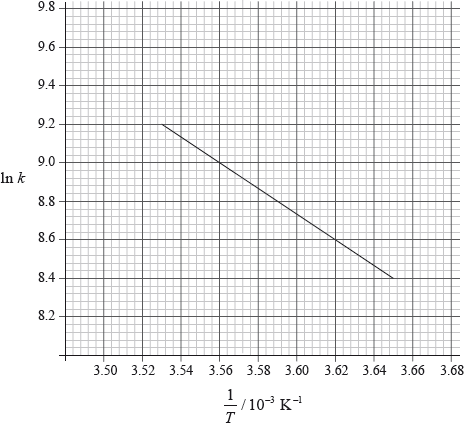
Consider the following graph of \(\ln k\) against \(\frac{1}{T}\) for the first order decomposition of \({{\text{N}}_{\text{2}}}{{\text{O}}_{\text{4}}}\) into \({\text{N}}{{\text{O}}_{\text{2}}}\). Determine the activation energy in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\) for this reaction.
Deduce the equilibrium constant expression, \({K_{\text{c}}}\), for the reaction.
Determine the value of the equilibrium constant, \({K_{\text{c}}}\).
If the temperature of the reaction is changed to 300 °C, predict, stating a reason in each case, whether the equilibrium concentration of \({\text{S}}{{\text{O}}_{\text{2}}}{\text{C}}{{\text{l}}_{\text{2}}}\) and the value of \({K_{\text{c}}}\) will increase or decrease.
If the volume of the container is changed to \({\text{1.50 d}}{{\text{m}}^{\text{3}}}\), predict, stating a reason in each case, how this will affect the equilibrium concentration of \({\text{S}}{{\text{O}}_2}{\text{C}}{{\text{l}}_2}\) and the value of \({K_{\text{c}}}\).
Suggest, stating a reason, how the addition of a catalyst at constant pressure and temperature will affect the equilibrium concentration of \({\text{S}}{{\text{O}}_{\text{2}}}{\text{C}}{{\text{l}}_{\text{2}}}\).
Markscheme
\({\text{rate}} = k{{\text{[NO]}}^2}{\text{[C}}{{\text{l}}_{\text{2}}}{\text{]}}\);
rate of reaction will decrease by a factor of 4;
no effect on the rate constant;
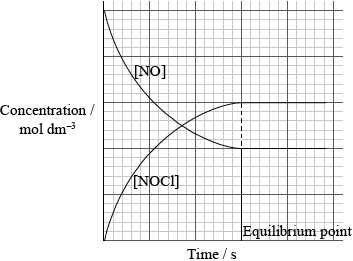
y axis labelled concentration/\({\text{mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) and x axis is labelled time/s;
gradient for [NO];
gradient for [NOCl] will be equal and opposite;
equilibrium point identified / two curves level off at same time;
Above 775 K: \({\text{rate}} = k{\text{[N}}{{\text{O}}_2}{\text{][CO]}}\);
Below 775 K: \({\text{rate}} = k{{\text{[N}}{{\text{O}}_2}{\text{]}}^2}\);
zero order reaction;
all concentrations are \({\text{1.0 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\);
\({\text{slope}} = \frac{{9.2 - 8.4}}{{(3.53 - 3.65) \times {{10}^{ - 3}}}} = - 6.67 \times {10^3}\);
\(({E_{\text{a}}} = 6.67 \times {10^3} \times 8.31)\)
\({\text{55.4 (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
Accept in range 55.0 – 56.0
Award [1] if 55454 (J) stated
Award [2] for the correct final answer
\(({K_{\text{c}}}) = \frac{{{\text{[S}}{{\text{O}}_2}{\text{C}}{{\text{l}}_2}{\text{]}}}}{{{\text{[C}}{{\text{l}}_2}{\text{][S}}{{\text{O}}_2}{\text{]}}}}\);
Ignore state symbols.
Square brackets [ ] required for the equilibrium expression.
\({\text{7.84}} \times {\text{1}}{{\text{0}}^{ - 3}}{\text{ mol of S}}{{\text{O}}_2}\) and \({\text{7.84}} \times {\text{1}}{{\text{0}}^{ - 3}}{\text{ mol of C}}{{\text{l}}_2}\);
\({\text{7.84}} \times {\text{1}}{{\text{0}}^{ - 3}}{\text{ mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{ of S}}{{\text{O}}_2}\), \({\text{7.84}} \times {\text{1}}{{\text{0}}^{ - 3}}{\text{ mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{ of C}}{{\text{l}}_2}\) and
\({\text{7.65}} \times {\text{1}}{{\text{0}}^{ - 4}}{\text{ mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{ of S}}{{\text{O}}_2}{\text{C}}{{\text{l}}_2}\);
12.5;
Award [1] for 10.34
Award [3] for the correct final answer
value of \({K_{\text{c}}}\) increases;
\({\text{[S}}{{\text{O}}_2}{\text{C}}{{\text{l}}_2}{\text{]}}\) increases;
decrease in temperature favours (forward) reaction which is exothermic;
Do not allow ECF.
no effect on the value of \({K_{\text{c}}}\) / depends only on temperature;
\({\text{[S}}{{\text{O}}_2}{\text{C}}{{\text{l}}_2}{\text{]}}\) decreases;
increase in volume favours the reverse reaction which has more gaseous moles;
Do not allow ECF.
no effect;
catalyst increases the rate of forward and reverse reactions (equally) / catalyst decreases activation energies (equally);
Examiners report
In part (a) the rate expression was correctly stated although some confused this with an equilibrium constant expression.
Only the better candidates realized that the rate of reaction will decrease by a factor of four and there will be no effect on the rate constant.
Although most candidates were able to correctly sketch the concentration versus time graph many forgot to label the axes or include units.
Part (b) was well answered and candidates demonstrated a good understanding of rate expressions based on reaction mechanism.
The better candidates were able to figure out that the rate of a chemical reaction is equal to the rate constant when all concentrations are \({\text{1.0 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) or for a zero order reaction.
Most candidates had difficulty in calculating activation energy from the graph in part (d) and some gave the answer in \({\text{J}}\,{\text{mo}}{{\text{l}}^{ - 1}}\) instead of \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\) which showed that they missed this instruction in the question.
In part (e), the equilibrium constant expression was correctly stated by the majority but calculating the value of\({K_{\text{c}}}\) proved to be difficult.
A large number of candidates obtained the incorrect answer of 10.34 as a result of using the initial concentrations of the reactants instead of equilibrium concentrations.
The application of Le Chatelier’s principle was handled well by the majority with minor omissions such as not using the term gaseous particles in part (iv).
Some candidates stated that the addition of a catalyst does not affect the value of \({K_{\text{c}}}\) or the position of equilibrium, which did not answer the question and scored no marks because they had not commented on the concentration of \({\text{SOC}}{{\text{l}}_{\text{2}}}\). Some candidates correctly stated that a catalyst increases the rate of forward and reverse reactions equally.
A group of students investigated the rate of the reaction between aqueous sodium thiosulfate and hydrochloric acid according to the equation below.
\[{\text{N}}{{\text{a}}_2}{{\text{S}}_2}{{\text{O}}_3}{\text{(aq)}} + {\text{2HCl(aq)}} \to {\text{2NaCl(aq)}} + {\text{S}}{{\text{O}}_2}{\text{(g)}} + {\text{S(s)}} + {{\text{H}}_2}{\text{O(l )}}\]
The two reagents were rapidly mixed together in a beaker and placed over a mark on a piece of paper. The time taken for the precipitate of sulfur to obscure the mark when viewed through the reaction mixture was recorded.

Initially they measured out \({\text{10.0 c}}{{\text{m}}^{\text{3}}}\) of \({\text{0.500 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) hydrochloric acid and then added \({\text{40.0 c}}{{\text{m}}^{\text{3}}}\) of \({\text{0.0200 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) aqueous sodium thiosulfate. The mark on the paper was obscured 47 seconds after the solutions were mixed.
One proposed mechanism for this reaction is:
\({{\text{S}}_2}{\text{O}}_3^{2 - }{\text{(aq)}} + {{\text{H}}^ + }{\text{(aq)}} \rightleftharpoons {\text{H}}{{\text{S}}_2}{\text{O}}_3^ - {\text{(aq)}}\) Fast
\({\text{H}}{{\text{S}}_2}{\text{O}}_3^ - {\text{(aq)}} + {{\text{H}}^ + }{\text{(aq)}} \to {\text{S}}{{\text{O}}_2}{\text{(g)}} + {\text{S(s)}} + {{\text{H}}_2}{\text{O(l)}}\) Slow
The teacher asked the students to devise another technique to measure the rate of this reaction.
Another group suggested collecting the sulfur dioxide and drawing a graph of the volume of gas against time.
(i) State the volumes of the liquids that should be mixed.
(ii) State why it is important that the students use a similar beaker for both reactions.
(iii) If the reaction were first order with respect to the thiosulfate ion, predict the time it would take for the mark on the paper to be obscured when the concentration of sodium thiosulfate solution is halved.
(i) Deduce the rate expression of this mechanism.
(ii) The results of an experiment investigating the effect of the concentration of hydrochloric acid on the rate, while keeping the concentration of thiosulfate at the original value, are given in the table below.
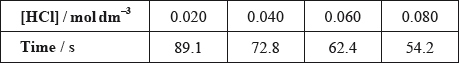
On the axes provided, draw an appropriate graph to investigate the order of the reaction with respect to hydrochloric acid.
(iii) Identify two ways in which these data do not support the rate expression deduced in part (i).
(i) Sketch and label, indicating an approximate activation energy, the Maxwell–Boltzmann energy distribution curves for two temperatures, \({T_1}\) and \(T2{\text{ }}({T_2} > {T_1})\), at which the rate of reaction would be significantly different.

(ii) Explain why increasing the temperature of the reaction mixture would significantly increase the rate of the reaction.
(i) One group suggested recording how long it takes for the pH of the solution to change by one unit. Calculate the initial pH of the original reaction mixture.
(ii) Deduce the percentage of hydrochloric acid that would have to be used up for the pH to change by one unit.
Calculate the volume of sulfur dioxide, in \({\text{c}}{{\text{m}}^{\text{3}}}\), that the original reaction mixture would produce if it were collected at \(1.00 \times {10^5}{\text{ Pa}}\) and 300 K.
Sulfur dioxide, a major cause of acid rain, is quite soluble in water and the equilibrium shown below is established.
\({\text{S}}{{\text{O}}_2}{\text{(aq)}} + {{\text{H}}_2}{\text{O(l)}} \rightleftharpoons {\text{HSO}}_3^ - {\text{(aq)}} + {{\text{H}}^ + }{\text{(aq)}}\)
Given that the \({K_{\text{a}}}\) for this equilibrium is \(1.25 \times {10^{ - 2}}{\text{ mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\), determine the pH of a \(2.00{\text{ mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) solution of sulfur dioxide.
Using Table 15 of the Data Booklet, identify an organic acid that is a stronger acid than sulfur dioxide.
Markscheme
(i) 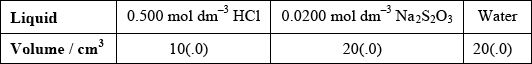 ;
;
Accept other volumes in a 1:2:2 ratio.
(ii) depth of liquid in the beaker must remain constant / OWTTE;
Accept “same thickness of glass” and any other valid point, such as answers framed around minimizing uncontrolled variables / making it a “fair test”.
(iii) 94 (s) / 1 min 34 s;
(i) \({\text{rate}} = k{\text{[}}{{\text{S}}_{\text{2}}}{\text{O}}_3^{2 - }{\text{][}}{{\text{H}}^ + }{{\text{]}}^2}/{\text{rate}} = k{\text{[N}}{{\text{a}}_2}{{\text{S}}_2}{{\text{O}}_3}{\text{][HCl}}{{\text{]}}^2}\);
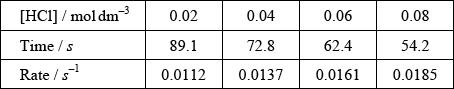
(ii) 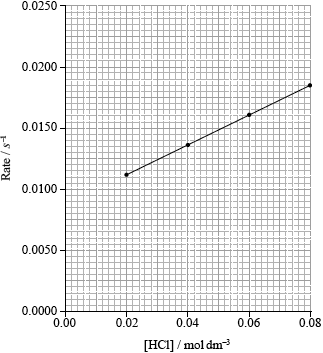
correct scale and units on y-axis;
Accept other suitable scales (such as 1/t) and units (such as ms–1).
Axes do not have to show origin/start at zero.
correct calculation of rate in \({s^{ - 1}}\);
If graph correct, assume this has been done on calculator and not written down.
correct plotting of points that the student decides to use and a connecting line;
Award final mark if 3 or more points are correct, irrespective of what is plotted on y-axis.
If line goes through the correct values at given concentrations of HCl, assume that points are marked there.
(iii) linear dependence on [HCl] (so not second order in \({\text{[}}{{\text{H}}^ + }{\text{]}}\));
Accept that doubling of concentration does not result in quadrupling of rate / OWTTE.
does not go through origin;
Remember to allow ECF from (b) (i).
(i) 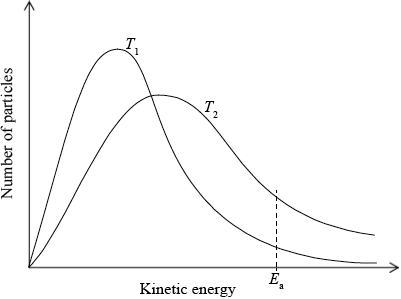
labelled y-axis: number of particles / probability of particles (with that kinetic energy) and labelled x-axis: (kinetic) energy;
Allow fraction/proportion/amount of particles (with kinetic energy) for y-axis label.
Allow speed/velocity for x-axis label.
\({T_2}\) curve broader and with maximum lower and to right of \({T_1}\) curve;
Do not award this mark if both curves not asymmetric.
Curves must pass through the origin and be asymptotic to x axis.
Do not award this mark if curves not labelled.
\({E_{\text{a}}}\) marked on graph;
(ii) kinetic energy of molecules increases;
This may be answered implicitly in the final marking point.
frequency of collision/number of collisions per unit time increases;
Do not accept “number of collisions increases”.
greater proportion of molecules have energy greater than/equal to activation energy / rate related to temperature by the Arrhenius equation;
Award [1 max] for statements such as “there will be more successful collisions” if neither of last two marking points awarded.
(i) \({\text{[}}{{\text{H}}^ + }{\text{]}} = 0.5 \times \frac{{10}}{{50}} = 0.1{\text{ (mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{)}}\);
\({\text{pH (}} = - \log {\text{[H}}{{\text{r}}^ + }{\text{]}} = - \log (0.10)) = 1\);
(ii) 90%;
\({\text{mol N}}{{\text{a}}_{\text{2}}}{{\text{S}}_{\text{2}}}{{\text{O}}_{\text{3}}} = {\text{mol S}}{{\text{O}}_{\text{2}}} = 0.0400 \times 0.0200 = 0.000800\);
\(V = \frac{{n \times R \times T}}{p}/\frac{{0.000800 \times 8.31 \times 300}}{{{{10}^5}}}\);
\((1.99 \times {10^{ - 5}}{\text{ }}{{\text{m}}^3}) = 19.9{\text{ }}({\text{c}}{{\text{m}}^3})\);
Note that two errors involving a factor of 1000 can also produce the correct answer. If this is the case award [1] not [3].
Accept 20.0 cm3 if R =8.314 is used.
Award [2] for 17.9 cm3 or 19.2 cm3 (result from using molar volume at standard temperature and pressure or at room temperature and pressure).
OR
\({\text{mol N}}{{\text{a}}_2}{{\text{S}}_2}{{\text{O}}_3} = {\text{mol S}}{{\text{O}}_2} = 0.0400 \times 0.0200 = 0.000800\);
\(V = 0.00080 \times 2.24 \times {10^{ - 2}} \times \left[ {\frac{{1.00 \times {{10}^5}}}{{1.01 \times {{10}^5}}}} \right] \times \frac{{300}}{{273}}\);
\((1.95 \times {10^{ - 5}}{\text{ }}{{\text{m}}^3}) = 19.5{\text{ }}({\text{c}}{{\text{m}}^3})\);
Note that two errors involving a factor of 1000 can also produce the correct answer. If this is the case award [1] not [3].
Deduct [1] for answers based on amount of HCl, so correct calculation would score [2 max].
\({K_{\text{a}}} = \frac{{{\text{[}}{{\text{H}}^ + }{\text{][HSO}}_3^ - {\text{]}}}}{{{\text{[}}{{\text{H}}_2}{\text{S}}{{\text{O}}_3}{\text{]}}}} = \frac{{{x^2}}}{{2 - x}} \approx \frac{{{x^2}}}{2} \approx 1.25 \times {10^{ - 2}}{\text{ (mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{)}}\);
\({\text{[}}{{\text{H}}^ + }{\text{]}} = \sqrt {2.50 \times {{10}^{ - 2}}} = 0.158{\text{ }}({\text{mol}}\,{\text{d}}{{\text{m}}^{ - 3}})\);
\({\text{pH}} = - \log (0.158) = 0.80\);
Award [3] for correct final answer.
dichloroethanoic acid / trichloroethanoic acid / 2,4,6-trinitrophenol;
Examiners report
This was quite a popular question, though generally not well answered. In the first part students again appeared to display a lack of expertise in a practical context with very few able to devise a mixture that would halve the concentration of thiosulfate, whilst keeping other concentrations constant, and answers predicting that this would halve the reaction time were far more commonly encountered than those doubling it. Many candidates did however suggest valid reasons why the reaction vessel should remain unchanged and a significant number of students were able to correctly deduce the rate equation that the mechanism given would predict. Again a lack of ability to interpret experimental data was evident in the fact that it was very rare to find students who realised that a graph of (time)-1 against concentration was required to be able to deduce the reaction order, with almost all simply plotting time-concentration graphs and, as a result, very few could evaluate the mechanism in the light of the experimental data. Part (c) was a fairly standard question on the effect of temperature on reaction rate, hence it was a surprise that students did not score better on it, with many of the oft repeated mistakes (number of collisions rather than collision frequency) again coming to the surface. Again it was probably inability to interpret experimental data that led to only very few students being able to correctly state the initial pH of the mixture (I am certain almost all would have gained the mark if the pH of \({\text{ 0.1 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) HCl had been asked for) and the percentage that would have to be consumed to increase the pH by one unit (which is independent of the previous answer) proved too much for almost all candidates. In part (e) most students could quote and substitute into the ideal gas equation, but converting from \({{\text{m}}^3}\) to \({\text{c}}{{\text{m}}^3}\) posed a problem for most candidates. Quite a number of candidates were however able to calculate the pH of the sulfur dioxide solution and identify a stronger acid.
This was quite a popular question, though generally not well answered. In the first part students again appeared to display a lack of expertise in a practical context with very few able to devise a mixture that would halve the concentration of thiosulfate, whilst keeping other concentrations constant, and answers predicting that this would halve the reaction time were far more commonly encountered than those doubling it. Many candidates did however suggest valid reasons why the reaction vessel should remain unchanged and a significant number of students were able to correctly deduce the rate equation that the mechanism given would predict. Again a lack of ability to interpret experimental data was evident in the fact that it was very rare to find students who realised that a graph of (time)-1 against concentration was required to be able to deduce the reaction order, with almost all simply plotting time-concentration graphs and, as a result, very few could evaluate the mechanism in the light of the experimental data. Part (c) was a fairly standard question on the effect of temperature on reaction rate, hence it was a surprise that students did not score better on it, with many of the oft repeated mistakes (number of collisions rather than collision frequency) again coming to the surface. Again it was probably inability to interpret experimental data that led to only very few students being able to correctly state the initial pH of the mixture (I am certain almost all would have gained the mark if the pH of \({\text{ 0.1 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) HCl had been asked for) and the percentage that would have to be consumed to increase the pH by one unit (which is independent of the previous answer) proved too much for almost all candidates. In part (e) most students could quote and substitute into the ideal gas equation, but converting from \({{\text{m}}^3}\) to \({\text{c}}{{\text{m}}^3}\) posed a problem for most candidates. Quite a number of candidates were however able to calculate the pH of the sulfur dioxide solution and identify a stronger acid.
This was quite a popular question, though generally not well answered. In the first part students again appeared to display a lack of expertise in a practical context with very few able to devise a mixture that would halve the concentration of thiosulfate, whilst keeping other concentrations constant, and answers predicting that this would halve the reaction time were far more commonly encountered than those doubling it. Many candidates did however suggest valid reasons why the reaction vessel should remain unchanged and a significant number of students were able to correctly deduce the rate equation that the mechanism given would predict. Again a lack of ability to interpret experimental data was evident in the fact that it was very rare to find students who realised that a graph of (time)-1 against concentration was required to be able to deduce the reaction order, with almost all simply plotting time-concentration graphs and, as a result, very few could evaluate the mechanism in the light of the experimental data. Part (c) was a fairly standard question on the effect of temperature on reaction rate, hence it was a surprise that students did not score better on it, with many of the oft repeated mistakes (number of collisions rather than collision frequency) again coming to the surface. Again it was probably inability to interpret experimental data that led to only very few students being able to correctly state the initial pH of the mixture (I am certain almost all would have gained the mark if the pH of \({\text{ 0.1 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) HCl had been asked for) and the percentage that would have to be consumed to increase the pH by one unit (which is independent of the previous answer) proved too much for almost all candidates. In part (e) most students could quote and substitute into the ideal gas equation, but converting from \({{\text{m}}^3}\) to \({\text{c}}{{\text{m}}^3}\) posed a problem for most candidates. Quite a number of candidates were however able to calculate the pH of the sulfur dioxide solution and identify a stronger acid.
This was quite a popular question, though generally not well answered. In the first part students again appeared to display a lack of expertise in a practical context with very few able to devise a mixture that would halve the concentration of thiosulfate, whilst keeping other concentrations constant, and answers predicting that this would halve the reaction time were far more commonly encountered than those doubling it. Many candidates did however suggest valid reasons why the reaction vessel should remain unchanged and a significant number of students were able to correctly deduce the rate equation that the mechanism given would predict. Again a lack of ability to interpret experimental data was evident in the fact that it was very rare to find students who realised that a graph of (time)-1 against concentration was required to be able to deduce the reaction order, with almost all simply plotting time-concentration graphs and, as a result, very few could evaluate the mechanism in the light of the experimental data. Part (c) was a fairly standard question on the effect of temperature on reaction rate, hence it was a surprise that students did not score better on it, with many of the oft repeated mistakes (number of collisions rather than collision frequency) again coming to the surface. Again it was probably inability to interpret experimental data that led to only very few students being able to correctly state the initial pH of the mixture (I am certain almost all would have gained the mark if the pH of \({\text{ 0.1 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) HCl had been asked for) and the percentage that would have to be consumed to increase the pH by one unit (which is independent of the previous answer) proved too much for almost all candidates. In part (e) most students could quote and substitute into the ideal gas equation, but converting from \({{\text{m}}^3}\) to \({\text{c}}{{\text{m}}^3}\) posed a problem for most candidates. Quite a number of candidates were however able to calculate the pH of the sulfur dioxide solution and identify a stronger acid.
This was quite a popular question, though generally not well answered. In the first part students again appeared to display a lack of expertise in a practical context with very few able to devise a mixture that would halve the concentration of thiosulfate, whilst keeping other concentrations constant, and answers predicting that this would halve the reaction time were far more commonly encountered than those doubling it. Many candidates did however suggest valid reasons why the reaction vessel should remain unchanged and a significant number of students were able to correctly deduce the rate equation that the mechanism given would predict. Again a lack of ability to interpret experimental data was evident in the fact that it was very rare to find students who realised that a graph of (time)-1 against concentration was required to be able to deduce the reaction order, with almost all simply plotting time-concentration graphs and, as a result, very few could evaluate the mechanism in the light of the experimental data. Part (c) was a fairly standard question on the effect of temperature on reaction rate, hence it was a surprise that students did not score better on it, with many of the oft repeated mistakes (number of collisions rather than collision frequency) again coming to the surface. Again it was probably inability to interpret experimental data that led to only very few students being able to correctly state the initial pH of the mixture (I am certain almost all would have gained the mark if the pH of \({\text{ 0.1 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) HCl had been asked for) and the percentage that would have to be consumed to increase the pH by one unit (which is independent of the previous answer) proved too much for almost all candidates. In part (e) most students could quote and substitute into the ideal gas equation, but converting from \({{\text{m}}^3}\) to \({\text{c}}{{\text{m}}^3}\) posed a problem for most candidates. Quite a number of candidates were however able to calculate the pH of the sulfur dioxide solution and identify a stronger acid.
This was quite a popular question, though generally not well answered. In the first part students again appeared to display a lack of expertise in a practical context with very few able to devise a mixture that would halve the concentration of thiosulfate, whilst keeping other concentrations constant, and answers predicting that this would halve the reaction time were far more commonly encountered than those doubling it. Many candidates did however suggest valid reasons why the reaction vessel should remain unchanged and a significant number of students were able to correctly deduce the rate equation that the mechanism given would predict. Again a lack of ability to interpret experimental data was evident in the fact that it was very rare to find students who realised that a graph of (time)-1 against concentration was required to be able to deduce the reaction order, with almost all simply plotting time-concentration graphs and, as a result, very few could evaluate the mechanism in the light of the experimental data. Part (c) was a fairly standard question on the effect of temperature on reaction rate, hence it was a surprise that students did not score better on it, with many of the oft repeated mistakes (number of collisions rather than collision frequency) again coming to the surface. Again it was probably inability to interpret experimental data that led to only very few students being able to correctly state the initial pH of the mixture (I am certain almost all would have gained the mark if the pH of \({\text{ 0.1 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) HCl had been asked for) and the percentage that would have to be consumed to increase the pH by one unit (which is independent of the previous answer) proved too much for almost all candidates. In part (e) most students could quote and substitute into the ideal gas equation, but converting from \({{\text{m}}^3}\) to \({\text{c}}{{\text{m}}^3}\) posed a problem for most candidates. Quite a number of candidates were however able to calculate the pH of the sulfur dioxide solution and identify a stronger acid.
This was quite a popular question, though generally not well answered. In the first part students again appeared to display a lack of expertise in a practical context with very few able to devise a mixture that would halve the concentration of thiosulfate, whilst keeping other concentrations constant, and answers predicting that this would halve the reaction time were far more commonly encountered than those doubling it. Many candidates did however suggest valid reasons why the reaction vessel should remain unchanged and a significant number of students were able to correctly deduce the rate equation that the mechanism given would predict. Again a lack of ability to interpret experimental data was evident in the fact that it was very rare to find students who realised that a graph of (time)-1 against concentration was required to be able to deduce the reaction order, with almost all simply plotting time-concentration graphs and, as a result, very few could evaluate the mechanism in the light of the experimental data. Part (c) was a fairly standard question on the effect of temperature on reaction rate, hence it was a surprise that students did not score better on it, with many of the oft repeated mistakes (number of collisions rather than collision frequency) again coming to the surface. Again it was probably inability to interpret experimental data that led to only very few students being able to correctly state the initial pH of the mixture (I am certain almost all would have gained the mark if the pH of \({\text{ 0.1 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) HCl had been asked for) and the percentage that would have to be consumed to increase the pH by one unit (which is independent of the previous answer) proved too much for almost all candidates. In part (e) most students could quote and substitute into the ideal gas equation, but converting from \({{\text{m}}^3}\) to \({\text{c}}{{\text{m}}^3}\) posed a problem for most candidates. Quite a number of candidates were however able to calculate the pH of the sulfur dioxide solution and identify a stronger acid.
Reaction kinetics can be investigated using the iodine clock reaction. The equations for two reactions that occur are given below.
Reaction A: \({{\text{H}}_2}{{\text{O}}_2}{\text{(aq)}} + {\text{2}}{{\text{I}}^ - }{\text{(aq)}} + {\text{2}}{{\text{H}}^ + }{\text{(aq)}} \to {{\text{I}}_2}{\text{(aq)}} + {\text{2}}{{\text{H}}_2}{\text{O(l)}}\)
Reaction B: \({\text{ }}{{\text{I}}_2}{\text{(aq)}} + {\text{2}}{{\text{S}}_2}{\text{O}}_3^{2 - }{\text{(aq)}} \to {\text{2}}{{\text{I}}^ - }{\text{(aq)}} + {{\text{S}}_4}{\text{O}}_6^{2 - }{\text{(aq)}}\)
Reaction B is much faster than reaction A, so the iodine, \({\text{I}_2}\), formed in reaction A immediately reacts with thiosulfate ions, \({{\text{S}}_{\text{2}}}{\text{O}}_3^{2 - }\), in reaction B, before it can react with starch to form the familiar blue-black, starch-iodine complex.
In one experiment the reaction mixture contained:
5.0 ± 0.1 \({\text{c}}{{\text{m}}^{\text{3}}}\) of 2.00 \({\text{mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) hydrogen peroxide (\({{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}\))
5.0 ± 0.1 \({\text{c}}{{\text{m}}^{\text{3}}}\) of 1% aqueous starch
20.0 ± 0.1 \({\text{c}}{{\text{m}}^{\text{3}}}\) of 1.00 \({\text{mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) sulfuric acid (\({{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}\))
20.0 ± 0.1 \({\text{c}}{{\text{m}}^{\text{3}}}\) of 0.0100 \({\text{mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) sodium thiosulfate (\({\text{N}}{{\text{a}}_{\text{2}}}{{\text{S}}_{\text{2}}}{{\text{O}}_{\text{3}}}\))
50.0 ± 0.1 \({\text{c}}{{\text{m}}^{\text{3}}}\) of water with 0.0200 ± 0.0001 g of potassium iodide (KI) dissolved in it.
After 45 seconds this mixture suddenly changed from colourless to blue-black.
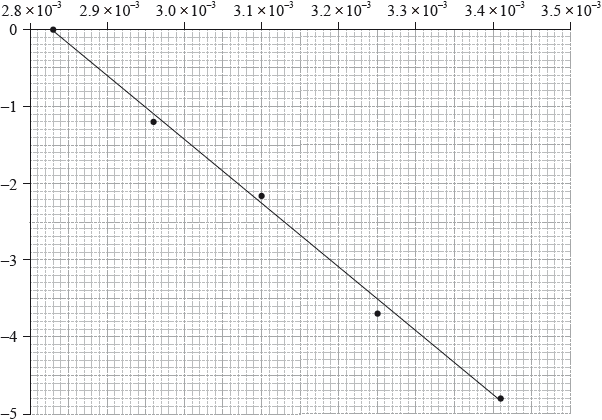
The activation energy can be determined using the Arrhenius equation, which is given in Table 1 of the Data Booklet. The experiment was carried out at five different temperatures. An incomplete graph to determine the activation energy of the reaction, based on these results, is shown below.
The concentration of iodide ions, \({{\text{I}}^ - }\), is assumed to be constant. Outline why this is a valid assumption.
For this mixture the concentration of hydrogen peroxide, \({{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}\), can also be assumed to be constant. Explain why this is a valid assumption.
Explain why the solution suddenly changes colour.
Calculate the total uncertainty, in \({\text{c}}{{\text{m}}^{\text{3}}}\), of the volume of the reaction mixture.
Calculate the percentage uncertainty of the concentration of potassium iodide solution added to the overall reaction mixture.
Determine the percentage uncertainty in the concentration of potassium iodide in the final reaction solution.
The colour change occurs when \(1.00 \times {10^{ - 4}}{\text{ mol}}\) of iodine has been formed. Use the total volume of the solution and the time taken, to calculate the rate of the reaction, including appropriate units.
State the labels for each axis.
x-axis:
y-axis:
Use the graph to determine the activation energy of the reaction, in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), correct to three significant figures.
In another experiment, 0.100 g of a black powder was also added while all other concentrations and volumes remained unchanged. The time taken for the solution to change colour was now 20 seconds. Outline why you think the colour change occurred more rapidly and how you could confirm your hypothesis.
Markscheme
KI/\({{\text{I}}^ - }\)/potassium iodide/iodide (ion) (rapidly) reformed (in second stage of reaction);
amount (in mol) of \({{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}\)/hydrogen peroxide \( \gg \) amount (in mol) \({\text{N}}{{\text{a}}_{\text{2}}}{{\text{S}}_{\text{2}}}{{\text{O}}_{\text{3}}}{\text{/}}{{\text{S}}_{\text{2}}}{\text{O}}_3^{2 - }\)/sodium thiosulfate/ thiosulfate (ion);
Accept amount (in mol) of H2O2/hydrogen peroxide \( \gg \) amount (in mol) KI/I–/potassium iodide/iodide (ion).
Accept “H2O2/hydrogen peroxide is in (large) excess/high concentration”.
(at end of reaction) \({\text{[}}{{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}{\text{]}}\) is only slightly decreased/virtually unchanged;
all \({\text{N}}{{\text{a}}_{\text{2}}}{{\text{S}}_{\text{2}}}{{\text{O}}_{\text{3}}}\)/sodium thiosulfate/\({{\text{S}}_{\text{2}}}{\text{O}}_3^{2 - }\)/thiosulfate consumed/used up;
Accept “iodine no longer converted to iodide”.
(free) iodine is formed / iodine reacts with starch / forms iodine-starch complex;
\((5 \times 0.1) = ( \pm )0.5{\text{ }}({\text{c}}{{\text{m}}^{\text{3}}})\);
\(( \pm )0.7(\% )\);
Comprises both mass of KI = ± 0.5% and volume of KI = ± 0.2%.
\(0.5 + 0.7 = ( \pm )1.2\% \);
Sum of (i) and (ii) (percentage uncertainty of total volume = absolute uncertainty as 100 cm3).
total volume \(0.100{\text{ }}({\text{d}}{{\text{m}}^3})/100{\text{ }}({\text{c}}{{\text{m}}^3})\);
\(\left( {{\text{change in concentration }} = \frac{{{\text{1.00}} \times {\text{1}}{{\text{0}}^{ - 4}}}}{{{\text{0.100}}}} = } \right){\text{ 1.00}} \times {\text{1}}{{\text{0}}^{ - 3}}{\text{ (mol}}\,{\text{d}}{{\text{m}}^{\text{3}}}{\text{)}}\);
\(\left( {{\text{rate}} = \frac{{1.00 \times {{10}^{ - 3}}}}{{45}} = } \right){\text{ }}2.2 \times {10^{ - 5}}\);
Award [3] for the correct final answer.
\({\text{mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{{\text{s}}^{ - 1}}\);
x-axis: \(\frac{1}{{{\text{Temperature}}}}/\frac{1}{T}/{{\text{T}}^{ - 1}}\);
Ignore units.
y-axis: ln rate/\({\log _{\text{e}}}\) rate / ln rate constant/\({\log _{\text{e}}}\) rate constant / ln k/\({\log _{\text{e}}}k\);
gradient \( = \frac{{ - {E_{\text{a}}}}}{R}\);
gradient \( = \frac{{ - 4.00}}{{(3.31 \times {{10}^{ - 3}} - 2.83 \times {{10}^{ - 3}})}} = - 8333/ = \frac{{ - 4.80}}{{(3.41 \times {{10}^{ - 3}} - 2.83 \times {{10}^{ - 3}})}} = - 8276\);
\({E_{\text{a}}} = \left( {\frac{{8.31 \times 8333}}{{1000}}} \right) = 69.3{\text{ }}({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}})/ = \left( {\frac{{8.31 \times 8276}}{{1000}}} \right) = 68.8{\text{ }}({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}})\);
Award [3] for correct final answer.
Accept values from 65.0 to 73.0 kJ mol–1.
Deduct [1] for final answer in J mol–1.
Deduct [1] for final answer not to 3 significant figures.
acting as a catalyst / black powder reacts with thiosulfate ions / solid dissolves to give blue-black solution;
Accept any other valid suggestion which will make colour change more rapid.
For catalyst: amount/mass of black powder remains constant / no new/different products formed / activation energy decreased;
For other suggestions: any appropriate way to test the hypothesis;
Award [1] for valid hypothesis, [1] for appropriate method of testing the stated hypothesis.
Examiners report
This question explored basic chemical concepts in the context of a practical situation. Whilst this is one frequently carried out during practical courses, none of the questions depended on prior knowledge. Students varied significantly in their ability to interpret the information given to answer parts (a) to (c), but very few could correctly carry out the propagation of uncertainties required in part (d). An encouraging number were able to carry out the rate calculation required in part (e). It was surprising how many students, though unable to identify the axes of the Arrhenius graph given in part (f), were still able to interpret it to correctly calculate the activation energy. Part (g) was deliberately open ended and elicited a number of interesting responses, though frequently the tests proposed would not in fact confirm the suggested hypothesis.
This question explored basic chemical concepts in the context of a practical situation. Whilst this is one frequently carried out during practical courses, none of the questions depended on prior knowledge. Students varied significantly in their ability to interpret the information given to answer parts (a) to (c), but very few could correctly carry out the propagation of uncertainties required in part (d). An encouraging number were able to carry out the rate calculation required in part (e). It was surprising how many students, though unable to identify the axes of the Arrhenius graph given in part (f), were still able to interpret it to correctly calculate the activation energy. Part (g) was deliberately open ended and elicited a number of interesting responses, though frequently the tests proposed would not in fact confirm the suggested hypothesis.
This question explored basic chemical concepts in the context of a practical situation. Whilst this is one frequently carried out during practical courses, none of the questions depended on prior knowledge. Students varied significantly in their ability to interpret the information given to answer parts (a) to (c), but very few could correctly carry out the propagation of uncertainties required in part (d). An encouraging number were able to carry out the rate calculation required in part (e). It was surprising how many students, though unable to identify the axes of the Arrhenius graph given in part (f), were still able to interpret it to correctly calculate the activation energy. Part (g) was deliberately open ended and elicited a number of interesting responses, though frequently the tests proposed would not in fact confirm the suggested hypothesis.
This question explored basic chemical concepts in the context of a practical situation. Whilst this is one frequently carried out during practical courses, none of the questions depended on prior knowledge. Students varied significantly in their ability to interpret the information given to answer parts (a) to (c), but very few could correctly carry out the propagation of uncertainties required in part (d). An encouraging number were able to carry out the rate calculation required in part (e). It was surprising how many students, though unable to identify the axes of the Arrhenius graph given in part (f), were still able to interpret it to correctly calculate the activation energy. Part (g) was deliberately open ended and elicited a number of interesting responses, though frequently the tests proposed would not in fact confirm the suggested hypothesis.
This question explored basic chemical concepts in the context of a practical situation. Whilst this is one frequently carried out during practical courses, none of the questions depended on prior knowledge. Students varied significantly in their ability to interpret the information given to answer parts (a) to (c), but very few could correctly carry out the propagation of uncertainties required in part (d). An encouraging number were able to carry out the rate calculation required in part (e). It was surprising how many students, though unable to identify the axes of the Arrhenius graph given in part (f), were still able to interpret it to correctly calculate the activation energy. Part (g) was deliberately open ended and elicited a number of interesting responses, though frequently the tests proposed would not in fact confirm the suggested hypothesis.
This question explored basic chemical concepts in the context of a practical situation. Whilst this is one frequently carried out during practical courses, none of the questions depended on prior knowledge. Students varied significantly in their ability to interpret the information given to answer parts (a) to (c), but very few could correctly carry out the propagation of uncertainties required in part (d). An encouraging number were able to carry out the rate calculation required in part (e). It was surprising how many students, though unable to identify the axes of the Arrhenius graph given in part (f), were still able to interpret it to correctly calculate the activation energy. Part (g) was deliberately open ended and elicited a number of interesting responses, though frequently the tests proposed would not in fact confirm the suggested hypothesis.
This question explored basic chemical concepts in the context of a practical situation. Whilst this is one frequently carried out during practical courses, none of the questions depended on prior knowledge. Students varied significantly in their ability to interpret the information given to answer parts (a) to (c), but very few could correctly carry out the propagation of uncertainties required in part (d). An encouraging number were able to carry out the rate calculation required in part (e). It was surprising how many students, though unable to identify the axes of the Arrhenius graph given in part (f), were still able to interpret it to correctly calculate the activation energy. Part (g) was deliberately open ended and elicited a number of interesting responses, though frequently the tests proposed would not in fact confirm the suggested hypothesis.
This question explored basic chemical concepts in the context of a practical situation. Whilst this is one frequently carried out during practical courses, none of the questions depended on prior knowledge. Students varied significantly in their ability to interpret the information given to answer parts (a) to (c), but very few could correctly carry out the propagation of uncertainties required in part (d). An encouraging number were able to carry out the rate calculation required in part (e). It was surprising how many students, though unable to identify the axes of the Arrhenius graph given in part (f), were still able to interpret it to correctly calculate the activation energy. Part (g) was deliberately open ended and elicited a number of interesting responses, though frequently the tests proposed would not in fact confirm the suggested hypothesis.
This question explored basic chemical concepts in the context of a practical situation. Whilst this is one frequently carried out during practical courses, none of the questions depended on prior knowledge. Students varied significantly in their ability to interpret the information given to answer parts (a) to (c), but very few could correctly carry out the propagation of uncertainties required in part (d). An encouraging number were able to carry out the rate calculation required in part (e). It was surprising how many students, though unable to identify the axes of the Arrhenius graph given in part (f), were still able to interpret it to correctly calculate the activation energy. Part (g) was deliberately open ended and elicited a number of interesting responses, though frequently the tests proposed would not in fact confirm the suggested hypothesis.
This question explored basic chemical concepts in the context of a practical situation. Whilst this is one frequently carried out during practical courses, none of the questions depended on prior knowledge. Students varied significantly in their ability to interpret the information given to answer parts (a) to (c), but very few could correctly carry out the propagation of uncertainties required in part (d). An encouraging number were able to carry out the rate calculation required in part (e). It was surprising how many students, though unable to identify the axes of the Arrhenius graph given in part (f), were still able to interpret it to correctly calculate the activation energy. Part (g) was deliberately open ended and elicited a number of interesting responses, though frequently the tests proposed would not in fact confirm the suggested hypothesis.
Consider the following graph of \(\ln k\) against \(\frac{1}{T}\).
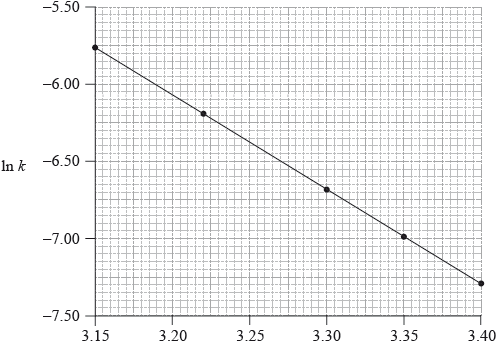
\[\frac{1}{T}/{10^{ - 3}}{\text{ }}{{\text{K}}^{ - 1}}\]
A catalyst provides an alternative pathway for a reaction, lowering the activation energy, \({E_{\text{a}}}\). Define the term activation energy, \({E_{\text{a}}}\).
State how the rate constant, k , varies with temperature, T.
Determine the activation energy, \({E_{\text{a}}}\), correct to three significant figures and state its units.
Markscheme
minimum energy needed (by reactants/colliding particles) to react/start/initiate a reaction / for a successful collision;
Allow energy difference between reactants and transition state.
k increases with T;
Do not accept k proportional to T or statement of Arrhenius equation from Data booklet.
slope/gradient/\(m = \frac{{ - {E_{\text{a}}}}}{R}/ - 6.20 \times {10^3}\);
Allow range of m from –5.96 \( \times \) 103 to –6.44 \( \times \) 103.
Award M1 for \(m = \frac{{ - {E_{\text{a}}}}}{R}\) even if gradient is out of range.
\({E_{\text{a}}} = (6.20 \times {10^3} \times 8.31) = 51.5{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}/5.15 \times {10^4}{\text{ J}}\,{\text{mo}}{{\text{l}}^{ - 1}}\)
\({E_{\text{a}}}\) value correct;
units correct;
Award [3] for correct final answer.
Allow range of Ea from 49.5 to 53.5 kJ\(\,\)mol–1 / 4.95 \( \times \) 104 to 5.35 \( \times \) 104 J\(\,\)mol–1.
Answer must be given correct to three significant figures.
M3 can be scored independently.
Examiners report
In (a) the most common mistake was for students to omit minimum in the definition of activation energy. Many described the relation between temperature and rate constant as linear or ‘proportional’. Only a small number of students gained full marks for the determination of activation energy because many either calculated an incorrect gradient or used the wrong units.
In (a) the most common mistake was for students to omit minimum in the definition of activation energy. Many described the relation between temperature and rate constant as linear or ‘proportional’. Only a small number of students gained full marks for the determination of activation energy because many either calculated an incorrect gradient or used the wrong units.
In (a) the most common mistake was for students to omit minimum in the definition of activation energy. Many described the relation between temperature and rate constant as linear or ‘proportional’. Only a small number of students gained full marks for the determination of activation energy because many either calculated an incorrect gradient or used the wrong units.
Alex and Hannah were asked to investigate the kinetics involved in the iodination of propanone. They were given the following equation by their teacher.
\[{\text{C}}{{\text{H}}_3}{\text{COC}}{{\text{H}}_3}{\text{(aq)}} + {{\text{I}}_2}{\text{(aq)}}\xrightarrow{{{{\text{H}}^ + }{\text{(aq)}}}}{\text{C}}{{\text{H}}_2}{\text{ICOC}}{{\text{H}}_3}{\text{(aq)}} + {\text{HI(aq)}}\]
Alex’s hypothesis was that the rate will be affected by changing the concentrations of the propanone and the iodine, as the reaction can happen without a catalyst. Hannah’s hypothesis was that as the catalyst is involved in the reaction, the concentrations of the propanone, iodine and the hydrogen ions will all affect the rate.
They carried out several experiments varying the concentration of one of the reactants or the catalyst whilst keeping other concentrations and conditions the same, and obtained the results below.
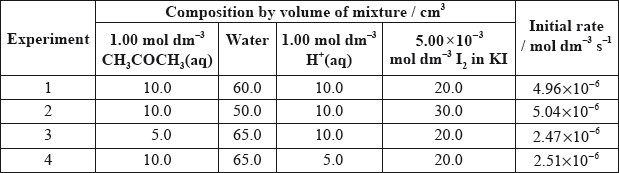
Explain why they added water to the mixtures.
(i) Deduce the order of reaction for each substance and the rate expression from the results.
(ii) Comment on whether Alex’s or Hannah’s hypothesis is correct.
Using the data from Experiment 1, determine the concentration of the substances used and the rate constant for the reaction including its units.
(i) This reaction uses a catalyst. Sketch and annotate the Maxwell-Boltzmann energy distribution curve for a reaction with and without a catalyst on labelled axes below.
(ii) Describe how a catalyst works.
Markscheme
to maintain a constant volume / OWTTE;
(i) \({\text{[}}{{\text{H}}^ + }{\text{]}}\) order 1, \({\text{[C}}{{\text{H}}_{\text{3}}}{\text{COC}}{{\text{H}}_{\text{3}}}{\text{]}}\) order 1, \({\text{[}}{{\text{I}}_{\text{2}}}{\text{]}}\) order 0;
\({\text{(rate}} = {\text{)}}k{\text{[}}{{\text{H}}^ + }{\text{][C}}{{\text{H}}_{\text{3}}}{\text{COC}}{{\text{H}}_{\text{3}}}{\text{]}}\);
Award [2] for correct rate expression.
Allow expressions including [I2]0.
(ii) neither were correct / Alex was right about propanone and wrong about iodine / Hannah was right about propanone and hydrogen ions but wrong about iodine / OWTTE;
\({\text{[C}}{{\text{H}}_{\text{3}}}{\text{COC}}{{\text{H}}_{\text{3}}}{\text{]}} = 0.100{\text{ mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) and \({\text{[}}{{\text{H}}^ + }{\text{]}} = 0.100{\text{ mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\);
\(k = \frac{{4.96 \times {{10}^{ - 6}}}}{{(0.100 \times 0.100)}} = 4.96 \times {10^{ - 4}}\);
\({\text{mo}}{{\text{l}}^{ - 1}}{\text{d}}{{\text{m}}^{\text{3}}}{{\text{s}}^{ - 1}}\);
Ignore calculation of [I2].
No ECF here for incorrect units.
(i) 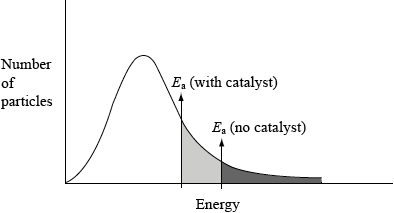
axes correctly labelled x = energy/velocity/speed, y = number/% of molecules/particles;
graph showing correct curve for Maxwell-Boltzmann distribution;
If two curves are drawn, first and second mark can still be scored, but not third.
Curve(s) must begin at origin and not go up at high energy.
two activation energies shown with \({E_{{\text{cat}}}}\) shown lower;
Award the mark for the final point if shown on an enthalpy level diagram.
(ii) catalyst provides an alternative pathway of lower energy / OWTTE;
Accept catalyst lowers activation energy (of reaction).
Examiners report
The presented data in the question proved to be quite tricky for many candidates, and answers to this question were generally disappointing. Very few stated the need to maintain a constant volume in (a) and many thought that water was added in order to provide a solvent for the reagents.
In (b)(i), although the question clearly told candidates to deduce the order for each substance, several did this for only two substances, often the species shown as reactants in the supplied equation. Then the orders shown in the rate expression did not always match the ones deduced. Only the better candidates got the rate expression correct and lots of guess work was seen here. A number gave \({K_c}\) instead of \(k\). The hypothesis question was also poorly answered and many candidates were not prepared for a question where both were incorrect.
Part (c) proved difficult and only the very best candidates got the two concentrations correct most just substituted volumes into their rate expression.
In (d), many candidates drew an enthalpy level diagram and not the Maxwell-Boltzmann distribution curve and others showed two curves. Those that did draw a correct curve often mislabelled the axes. However, the vast majority could explain how a catalyst worked.
Sodium thiosulfate solution, \({\text{N}}{{\text{a}}_{\text{2}}}{{\text{S}}_{\text{2}}}{{\text{O}}_{\text{3}}}{\text{(aq)}}\), and hydrochloric acid, \({\text{HCl(aq)}}\), react to produce solid sulfur as in the equation below.
\[{{\text{S}}_2}{\text{O}}_3^{2 - }{\text{(aq)}} + {\text{2}}{{\text{H}}^ + }{\text{(aq)}} \to {\text{S(s)}} + {\text{S}}{{\text{O}}_2}{\text{(g)}} + {{\text{H}}_2}{\text{O(l)}}\]
The following results to determine the initial rate were obtained:
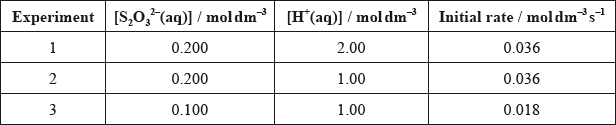
Deduce, with a reason, the order of reaction with respect to each reactant.
State the rate expression for this reaction.
Determine the value of the rate constant, \(k\), and state its units.
State an equation for a possible rate-determining step for the reaction.
Suggest how the activation energy, \({E_{\text{a}}}\), for this reaction may be determined.
Markscheme
experiments 1 and 2 (\({\text{[}}{{\text{S}}_2}{\text{O}}_3^{2 - }{\text{]}}\) remains constant) change in \({\text{[}}{{\text{H}}^ + }{\text{]}}\) does not affect the rate so zero order with respect to \({{\text{H}}^ + }{\text{(aq)}}\) / OWTTE;
experiment 1/2 and 3 (\({\text{[}}{{\text{H}}^ + }{\text{]}}\) has no effect) \({\text{[}}{{\text{S}}_2}{\text{O}}_3^{2 - }{\text{]}}\) is halved and rate is also halved so first order with respect to \({\text{[}}{{\text{S}}_2}{\text{O}}_3^{2 - }{\text{]}}\) / OWTTE;
Accept explanation given in mathematical terms.
Award [1 max] if both [S2O32–] is first order, and [H+] is zero order are stated without reason.
rate \( = k{\text{[}}{{\text{S}}_2}{\text{O}}_3^{2 - }{\text{]}}\);
0.18;
\({{\text{s}}^{ - 1}}\);
\({{\text{S}}_2}{\text{O}}_3^{2 - } \to {\text{S}} + {\text{SO}}_3^{2 - }\);
Accept any balanced equation that starts with only one S2O32–.
Equations must be balanced in terms of number of atoms and charge.
determine rate at a range of temperatures (while keeping concentrations constant);
calculate \(k\) for each temperature;
plot graph of \(\ln k\) against \({T^{ - {\text{1}}}}\);
gradient is \(\frac{{ - {E_{\text{a}}}}}{R}/\)OWTTE;
Examiners report
The interpretation of orders of rate from experimental data was well understood, and explained. Calculations of both the value and units of \({K_{\text{c}}}\) were also done well. Very few candidates produced an acceptable equation for the rate determining step, many did not realise the importance of balancing both the number of atoms and charge on both sides. The required careful explanation of how \({E_{\text{a}}}\) is determined from experimental data was lacking, too often a vague description of using gradient and \(R\) without context was considered sufficient by many candidates.
The interpretation of orders of rate from experimental data was well understood, and explained. Calculations of both the value and units of \({K_{\text{c}}}\) were also done well. Very few candidates produced an acceptable equation for the rate determining step, many did not realise the importance of balancing both the number of atoms and charge on both sides. The required careful explanation of how \({E_{\text{a}}}\) is determined from experimental data was lacking, too often a vague description of using gradient and \(R\) without context was considered sufficient by many candidates.
The interpretation of orders of rate from experimental data was well understood, and explained. Calculations of both the value and units of \({K_{\text{c}}}\) were also done well. Very few candidates produced an acceptable equation for the rate determining step, many did not realise the importance of balancing both the number of atoms and charge on both sides. The required careful explanation of how \({E_{\text{a}}}\) is determined from experimental data was lacking, too often a vague description of using gradient and \(R\) without context was considered sufficient by many candidates.
The interpretation of orders of rate from experimental data was well understood, and explained. Calculations of both the value and units of \({K_{\text{c}}}\) were also done well. Very few candidates produced an acceptable equation for the rate determining step, many did not realise the importance of balancing both the number of atoms and charge on both sides. The required careful explanation of how \({E_{\text{a}}}\) is determined from experimental data was lacking, too often a vague description of using gradient and \(R\) without context was considered sufficient by many candidates.
The interpretation of orders of rate from experimental data was well understood, and explained. Calculations of both the value and units of \({K_{\text{c}}}\) were also done well. Very few candidates produced an acceptable equation for the rate determining step, many did not realise the importance of balancing both the number of atoms and charge on both sides. The required careful explanation of how \({E_{\text{a}}}\) is determined from experimental data was lacking, too often a vague description of using gradient and \(R\) without context was considered sufficient by many candidates.
The Haber process enables the large-scale production of ammonia needed to make fertilizers.
The equation for the Haber process is given below.
\[{{\text{N}}_2}({\text{g)}} + 3{{\text{H}}_2}({\text{g)}} \rightleftharpoons {\text{2N}}{{\text{H}}_3}({\text{g)}}\]
The percentage of ammonia in the equilibrium mixture varies with temperature.
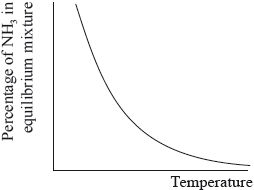
Ammonia can be converted into nitric acid, \({\text{HN}}{{\text{O}}_{\text{3}}}{\text{(aq)}}\), and hydrocyanic acid, HCN(aq). The \({\text{p}}{K_{\text{a}}}\) of hydrocyanic acid is 9.21.
A student decided to investigate the reactions of the two acids with separate samples of \({\text{0.20 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) sodium hydroxide solution.
(i) Use the graph to deduce whether the forward reaction is exothermic or endothermic and explain your choice.
(ii) State and explain the effect of increasing the pressure on the yield of ammonia.
(iii) Deduce the equilibrium constant expression, \({K_{\text{c}}}\), for the reaction.
(iv) A mixture of 1.00 mol \({{\text{N}}_{\text{2}}}\) and 3.00 mol \({{\text{H}}_{\text{2}}}\) was placed in a \({\text{1.0 d}}{{\text{m}}^{\text{3}}}\) flask at 400 °C. When the system was allowed to reach equilibrium, the concentration of was found to be \({\text{0.062 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\). Determine the equilibrium constant, \({K_{\text{c}}}\), of the reaction at this temperature.
(v) Iron is used as a catalyst in the Haber process. State the effect of a catalyst on the value of \({K_{\text{c}}}\).
(i) Distinguish between the terms strong and weak acid and state the equations used to show the dissociation of each acid in aqueous solution.
(ii) Deduce the expression for the ionization constant, \({K_{\text{a}}}\), of hydrocyanic acid and calculate its value from the \({\text{p}}{K_{\text{a}}}\) value given.
(iii) Use your answer from part (b) (ii) to calculate the \({\text{[}}{{\text{H}}^ + }{\text{]}}\) and the pH of an aqueous solution of hydrocyanic acid of concentration \({\text{0.108 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\). State one assumption made in arriving at your answer.
A small piece of magnesium ribbon is added to solutions of nitric and hydrocyanic acid of the same concentration at the same temperature. Describe two observations that would allow you to distinguish between the two acids.
(i) Calculate the volume of the sodium hydroxide solution required to react exactly with a \({\text{15.0 c}}{{\text{m}}^{\text{3}}}\) solution of \({\text{0.10 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) nitric acid.
(ii) The following hypothesis was suggested by the student: “Since hydrocyanic acid is a weak acid it will react with a smaller volume of the \({\text{0.20 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) sodium hydroxide solution.” Comment on whether or not this is a valid hypothesis.
(iii) Use Table 16 of the Data Booklet to identify a suitable indicator for the titration of sodium hydroxide and hydrocyanic acid.
Markscheme
(i) exothermic;
Accept either of the following for the second mark.
increasing temperature favours endothermic/reverse reaction;
as yield decreases with increasing temperature;
(ii) yield increases / equilibrium moves to the right / more ammonia;
increase in pressure favours the reaction which has fewer moles of gaseous products;
(iii) \({K_{\text{c}}} = \frac{{{{{\text{[N}}{{\text{H}}_3}{\text{]}}}^2}}}{{{\text{[}}{{\text{N}}_2}{\text{][}}{{\text{H}}_2}{{\text{]}}^3}}}\);
(iv) \({\text{[}}{{\text{N}}_2}{\text{]}}\): (at equilibrium \( = 1.00 - 0.031 = \)) \({\text{0.969 (mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{)}}\);
\({\text{[}}{{\text{H}}_2}{\text{]}}\): (at equilibrium \( = 3.00 - 3(0.031) = \)) \({\text{2.91 (mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{)}}\);
\({K_{\text{c}}}{\text{ }}\left( { = \frac{{{{{\text{(0.062)}}}^2}}}{{{\text{(0.969) (2.91}}{{\text{)}}^3}}}} \right) = {\text{1.6(1)}} \times {\text{1}}{{\text{0}}^{ - 4}}\);
Ignore units.
Award [1] for Kc = 1.4 \( \times \) 10–4
(v) no effect;
(i) strong acid completely dissociated/ionized and weak acid partially dissociated/ionized;
\({\text{HN}}{{\text{O}}_3}{\text{(aq)}} \to {{\text{H}}^ + }{\text{(aq)}} + {\text{NO}}_3^ - {\text{(aq)}}\);
\({\text{HCN(aq)}} \rightleftharpoons {{\text{H}}^ + }{\text{(aq)}} + {\text{C}}{{\text{N}}^ - }{\text{(aq)}}\);
Insist on both arrows as shown.
State symbols not needed.
Accept H2O and H3O+.
(ii) \({K_{\text{a}}} = \frac{{{\text{[}}{{\text{H}}^ + }{\text{][C}}{{\text{N}}^ - }{\text{]}}}}{{{\text{[HCN]}}}}\);
Allow H3O+ instead of H+.
\({K_{\text{a}}} = {10^{ - 9.21}} = 6.17 \times {10^{ - 10}}\);
(iii) \({[{{\text{H}}^ + }] = \sqrt {{K_{\text{a}}}[{\text{HCN}}]} /\sqrt {(6.17 \times {{10}^{ - 10}} \times 0.108)} }\);
\({ = 8.16 \times {{10}^{ - 6}}}\);
Allow in the range 8.13 \( \times \) 10–6 to 8.16 \( \times \) 10–6.
\({\text{pH}} = 5.09\);
OR
\({{\text{pH}} = \frac{1}{2}{\text{(p}}{K_{\text{a}}} - {\text{log}}[{\text{HCN}}])/\frac{1}{2}(9.21 - \log \,0.108)}\);
\({ = 5.09}\);
\({\text{[}}{{\text{H}}^ + }{\text{]}} = {10^{ - 5.09}} = 8.16 \times {10^{ - 6}}\);
Allow in the range 8.13 \( \times \) 10–6 to 8.16 \( \times \) 10–6.
If expression for [H+] missing but both answers correct, award [3], if one answer
correct, award [2].
assume \({\text{[}}{{\text{H}}^ + }{\text{]}} \ll 0.108\) / negligible dissociation;
With HNO3:
faster rate of bubble/hydrogen/gas production;
faster rate of magnesium dissolving;
higher temperature change;
Accept opposite argument for HCN.
Reference to specific observations needed.
Award [1] if 2 observations given but acid is not identified.
(i) (nitric acid) 7.5 cm3;
(ii) not valid as hydrocyanic acid reacts with same volume/ 7.5 cm3;
(iii) bromothymol blue / phenol red / phenolphthalein;
Examiners report
Equilibrium is a topic that has shown substantial improvement in recent sessions with some very well produced arguments. The reaction was correctly described as exothermic with a reason correctly given in most cases. Most candidates knew that yield would increase with increased pressure, but some failed to identify the change in the number of “gaseous” molecules as the reason. More candidates had difficulty with the equilibrium constant calculation often using the initial not equilibrium concentrations.
In (b) most correctly defined strong and weak acids and many also wrote correct equations. A few, however, missed the equilibrium sign for hydrocyanic acid. HA, CH3COOH and HCl were commonly given instead of HCN and HNO3, suggesting that students sometimes have difficulty applying general concepts to specific cases. It was encouraging to see many candidates determine the pH from the pKa value including the assumption that there is negligible dissociation, as this has challenged students in previous sessions. A significant number of weaker candidates reported however that the acid solution would have pH values above 7.
Part (c) presented problems with many candidates unable to describe specific observations related to rate which would distinguish between a strong and weak acid and simply stated that the reaction would be faster.
The moles calculation was answered well in (d) with most candidates able to identify phenolphthalein as a suitable indicator.
Chemical kinetics involves an understanding of how the molecular world changes with time.
A catalyst provides an alternative pathway for a reaction, lowering the activation energy, \({E_{\text{a}}}\).
Sketch graphical representations of the following reactions, for X \( \to \) products.
For the reaction below, consider the following experimental data.
\[{\text{2Cl}}{{\text{O}}_2}{\text{(aq)}} + {\text{2O}}{{\text{H}}^ - }{\text{(aq)}} \to {\text{ClO}}_3^ - {\text{(aq)}} + {\text{ClO}}_2^ - {\text{(aq)}} + {{\text{H}}_2}{\text{O(l)}}\]
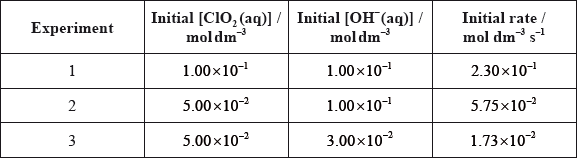
Another reaction involving \({\rm{O}}{{\rm{H}}^ - }\) (aq) is the base hydrolysis reaction of an ester.
\[{\text{C}}{{\text{H}}_3}{\text{COOC}}{{\text{H}}_2}{\text{CH(aq)}} + {\text{O}}{{\text{H}}^ - }{\text{(aq)}} \to {\text{C}}{{\text{H}}_3}{\text{CO}}{{\text{O}}^ - }{\text{(aq)}} + {\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{OH(aq)}}\]
A two-step mechanism has been proposed for the following reaction.
\[\begin{array}{*{20}{l}} {{\text{Step 1:}}}&{{\text{Cl}}{{\text{O}}^ - }{\text{(aq)}} + {\text{Cl}}{{\text{O}}^ - }{\text{(aq)}} \to {\text{ClO}}_2^ - {\text{(aq)}} + {\text{C}}{{\text{l}}^ - }{\text{(aq)}}} \\ {{\text{Step 2:}}}&{{\text{ClO}}_2^ - {\text{(aq)}} + {\text{Cl}}{{\text{O}}^ - }{\text{(aq)}} \to {\text{ClO}}_3^ - {\text{(aq)}} + {\text{C}}{{\text{l}}^ - }{\text{(aq)}}} \end{array}\]
(i) Define the term rate of reaction.
(ii) Temperature and the addition of a catalyst are two factors that can affect the rate of a reaction. State two other factors.
(iii) In the reaction represented below, state one method that can be used to measure the rate of the reaction.
\[{\text{ClO}}_3^ - {\text{(aq)}} + {\text{5C}}{{\text{l}}^ - }{\text{(aq)}} + {\text{6}}{{\text{H}}^ + }{\text{(aq)}} \to {\text{3C}}{{\text{l}}_2}{\text{(aq)}} + {\text{3}}{{\text{H}}_2}{\text{O(l)}}\]
(i) Define the term activation energy, \({E_{\text{a}}}\).
(ii) Sketch the two Maxwell–Boltzmann energy distribution curves for a fixed amount of gas at two different temperatures, \({T_1}\) and \({T_2}{\text{ }}({T_2} > {T_1})\). Label both axes.

(i) Concentration of reactant X against time for a zero-order reaction.
(ii) Rate of reaction against concentration of reactant X for a zero-order reaction.
(iii) Rate of reaction against concentration of reactant X for a first-order reaction.
(i) Deduce the rate expression.
(ii) Determine the rate constant, \(k\), and state its units, using the data from Experiment 2.
(iii) Calculate the rate, in \({\text{mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{{\text{s}}^{ - 1}}\), when \({\text{[Cl}}{{\text{O}}_2}{\text{(aq)]}} = 1.50 \times {10^{ - 2}}{\text{ mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) and \({\text{[O}}{{\text{H}}^ - }{\text{(aq)]}} = 2.35 \times {10^{ - 2}}{\text{ mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\).
Apply IUPAC rules to name the ester, CH3COOCH2CH3(aq).
Describe qualitatively the relationship between the rate constant, k, and temperature, T.
The rate of this reaction was measured at different temperatures and the following data were recorded.
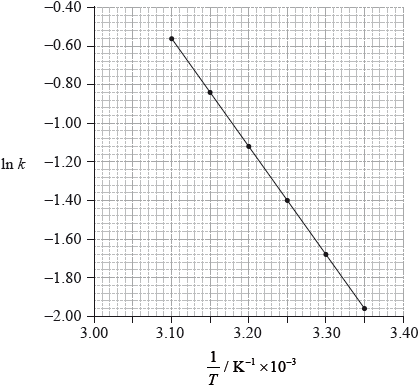
Using data from the graph, determine the activation energy, \({E_{\text{a}}}\), correct to three significant figures and state its units.
Deduce the overall equation for the reaction.
Deduce the rate expression for each step.
Step 1:
Step 2:
Markscheme
(i) change in concentration of reactant/product with time / rate of change of concentration;
Increase can be used instead of change for product or decrease can be used instead of change for reactant.
Allow mass/amount/volume instead of concentration.
Do not accept substance.
(ii) concentration;
particle size / surface area;
light;
pressure;
Allow pH.
(iii) (measuring electrical) conductivity / (measuring) pH;
Accept other suitable method.
(i) minimum/least/smallest energy needed (by reactants/colliding particles) to react/start/initiate a reaction;
Allow energy difference between reactants and transition state.
Minimum/least/smallest required for the mark.
(ii) x-axis label: (kinetic) energy/(K)E and y-axis label: probability/fraction of molecules/particles / probability density;
Allow number of molecules/particles for y-axis.
correct shape of a typical Maxwell–Boltzmann energy distribution curve;
Do not award mark if curve is symmetric, does not start at zero or if it crosses x-axis.
two curves represented with second curve for \({T_2} > {T_1}\) to right of first curve, peak maximum lower than first curve and after the curves cross going to the right, \({T_2}\) curve needs to be above \({T_1}\) curve as illustrated;
M2 and M3 can be scored independently.
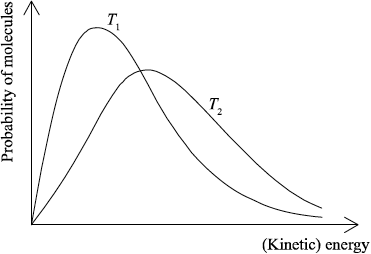
(i)  ;
;
(ii)  ;
;
(iii)  ;
;
(i) second order in \({\text{Cl}}{{\text{O}}_2}\) and first order in \({\text{O}}{{\text{H}}^ - }\);
rate \( = k{{\text{[Cl}}{{\text{O}}_{\text{2}}}{\text{]}}^2}{\text{[O}}{{\text{H}}^ - }{\text{]}}\);
Award [2] for correct final answer.
(ii) \(k = 2.30 \times {10^2}/230\);
\({\text{mo}}{{\text{l}}^{ - 2}}{\text{d}}{{\text{m}}^6}{{\text{s}}^{ - 1}}\);
(iii) \(1.22 \times {10^{ - 3}}/0.00122{\text{ (mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{{\text{s}}^{ - 1}}{\text{)}}\);
ethyl ethanoate;
Do not allow ethyl acetate.
as temperature/T increases, (value of) rate constant/k increases (exponentially);
Do not allow answers involving ln k from the Arrhenius equation.
Do not allow T directly proportional to k.
slope \( = - 5.6 \times {10^3}/ - 5600{\text{ (K)}}\);
\({E_{\text{a}}} = - {\text{slope}} \times {\text{R}}/{\text{slope}} = - {E_{\text{a}}}/R\);
\({E_{\text{a}}}{\text{(}} = 5.60 \times {10^3}{\text{ }}K \times 8.31{\text{ J}}\,{{\text{K}}^{ - 1}}{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}} = 4.65 \times {10^4}{\text{ (J}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}/46.5{\text{ (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
Accept answers in range 4.60 \( \times \) 104 J\(\,\)mol\(^{ - 1}\) to 4.67 \( \times \) \({10^4}\) (J mol \(^{ - 1}\)).
\({\text{J}}\,{\text{mo}}{{\text{l}}^{ - 1}}/{\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\);
Accept J or kJ.
Unit mark can be scored independently but correct \({E_a}\) values with incorrect units scores only [3 max] (for example 46.5 J mol \(^{ - 1}\)).
Award [4] for correct final answer.
\({\text{3Cl}}{{\text{O}}^ - }{\text{(aq)}} \to {\text{ClO}}_3^ - {\text{(aq)}} + {\text{2C}}{{\text{l}}^ - }{\text{(aq)}}\);
Ignore state symbols.
Step 1: rate \( = k{{\text{[Cl}}{{\text{O}}^ - }{\text{]}}^{\text{2}}}\);
Step 2: rate \( = k{\text{[ClO}}_2^ - {\text{][Cl}}{{\text{O}}^ - }{\text{]}}\);
Penalize missing k once only.
Examiners report
This was the most popular question in Section B of the paper. Part (a) was very well answered.
In (b) (i), some candidates failed to mention minimum/least/smallest energy in the definition of activation energy. In part (ii), again candidates often dropped easy marks here for poor representations of the Maxwell-Boltzmann energy distribution curves. In some cases the curves were drawn symmetrically, which was incorrect. In addition, incorrect labels were often given for the x- and y-axes. Some candidates mixed these curves up with enthalpy level diagrams. It was nice to see more candidates giving a more precise label for the y-axis as probability/fraction of molecules rather than just number of molecules. The latter was allowed but is less precise (although does tend to be used in many IB textbooks).
Part (c) however was very well answered.
In part (d), many candidates also scored highly though the units of k in (ii) did cause a problem for some candidates.
In (e) (i), the most common mistake was candidates stating ethyl methanoate instead of ethyl ethanoate.
In part (ii), a number of candidates stated incorrectly that T is directly proportional to k, which is incorrect. Proportionality is a concept embedded in AS 11.3.1 in Topic 11, and may be worth some further discussion in the light of the Arrhenius Equation.
The most difficult part of Q6 however involved (e) (iii). Very few candidates scored full marks here and simply did not know how to manipulate the equation to get the activation energy. Others even gave incorrect units.
One respondent stated that part (f) (ii) would be difficult for candidates. (f) certainly did prove challenging and the rate expression for step two was often given incorrectly. This question became a good discriminating question in Section B. However the better students did manage to score all three marks in part (f).
One respondent stated that part (f) (ii) would be difficult for candidates. (f) certainly did prove challenging and the rate expression for step two was often given incorrectly. This question became a good discriminating question in Section B. However the better students did manage to score all three marks in part (f).
Hydrogen peroxide decomposes according to the equation below.
\({\text{2}}{{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}{\text{(aq)}} \to {\text{2}}{{\text{H}}_{\text{2}}}{\text{O(l)}} + {{\text{O}}_{\text{2}}}{\text{(g)}}\)
The rate of the decomposition can be monitored by measuring the volume of oxygen gas released. The graph shows the results obtained when a solution of hydrogen peroxide decomposed in the presence of a CuO catalyst.
Outline how the initial rate of reaction can be found from the graph.
Explain how and why the rate of reaction changes with time.
A Maxwell-Boltzmann energy distribution curve is drawn below. Label both axes and explain, by annotating the graph, how catalysts increase the rate of reaction.
(i) In some reactions, increasing the concentration of a reactant does not increase the rate of reaction. Describe how this may occur.
(ii) Consider the reaction
\[{\text{2A}} + {\text{B}} \to {\text{C}} + {\text{D}}\]
The reaction is first order with respect to A, and zero order with respect to B. Deduce the rate expression for this reaction.
Sketch a graph of rate constant \((k)\) versus temperature.
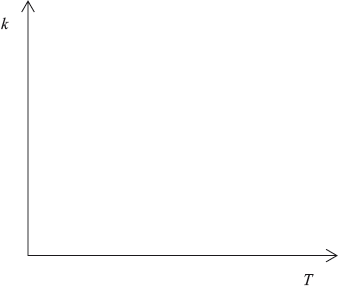
Hydrochloric acid neutralizes sodium hydroxide, forming sodium chloride and water.
\({\text{NaOH(aq)}} + {\text{HCl(aq)}} \to {\text{NaCl(aq)}} + {{\text{H}}_{\text{2}}}{\text{O(l)}}\) \(\Delta {H^\Theta } = - 57.9{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\)
(i) Define standard enthalpy change of reaction, \(\Delta {H^\Theta }\).
(ii) Determine the amount of energy released, in kJ, when \({\text{50.0 c}}{{\text{m}}^{\text{3}}}\) of \({\text{1.00 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) sodium hydroxide solution reacts with \({\text{50.0 c}}{{\text{m}}^{\text{3}}}\) of \({\text{1.00 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) hydrochloric acid solution.
(iii) In an experiment, 2.50 g of solid sodium hydroxide was dissolved in \({\text{50.0 c}}{{\text{m}}^{\text{3}}}\) of water. The temperature rose by 13.3 °C. Calculate the standard enthalpy change, in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), for dissolving one mole of solid sodium hydroxide in water.
\[{\text{NaOH(s)}} \to {\text{NaOH(aq)}}\]
(iv) Using relevant data from previous question parts, determine \(\Delta {H^\Theta }\), in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), for the reaction of solid sodium hydroxide with hydrochloric acid.
\[{\text{NaOH(s)}} + {\text{HCl(aq)}} \to {\text{NaCl(aq)}} + {{\text{H}}_{\text{2}}}{\text{O(l)}}\]
(i) Zinc is found in the d-block of the periodic table. Explain why it is not considered a transition metal.
(ii) Explain why \({\text{F}}{{\text{e}}^{3 + }}\) is a more stable ion than \({\text{F}}{{\text{e}}^{2 + }}\) by reference to their electron configurations.
Markscheme
(draw a) tangent to the curve at origin/time = 0/start of reaction;
(calculate) the gradient/slope (of the tangent);
rate decreases (with time);
concentration/number of (reactant) molecules per unit volume decreases (with time);
Do not accept “number of molecules decreases” or “amount of reactant decreases”.
collisions (between reactant molecules/reactant and catalyst) become less frequent;
Do not accept “fewer collisions” without reference to frequency (eg, no. collisions per second).
y-axis: probability / fraction of molecules/particles / probability density
Allow “number of particles/molecules” on y-axis.
and
x-axis: (kinetic) energy;
Accept “speed/velocity” on x-axis.
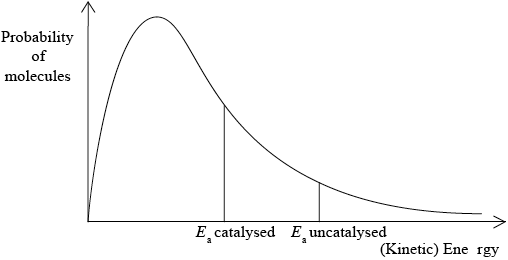
correct relative position of \({E_{\text{a}}}\) catalysed and \({E_{\text{a}}}\) uncatalysed;
more/greater proportion of molecules/collisions have the lower/required/catalysed \({E_{\text{a}}}\) (and can react upon collision);
M3 can be scored by stating or shading and annotating the graph.
Accept “a greater number/proportion of successful collisions as catalyst reduces \({E_a}\)”.
(i) reactant not involved in (or before) the slowest/rate-determining step/RDS;
reactant is in (large) excess;
(ii) \({\text{(rate}} = {\text{) }}k{\text{[A]}}\);
Accept rate = k[A]1[B]0.
curve with a positive slope curving upwards;
Do not penalize if curve passes through the origin.
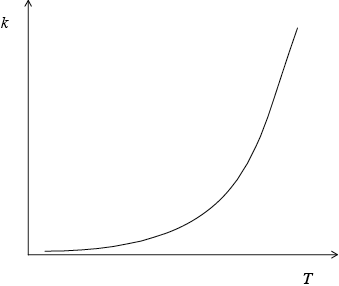
(i) heat transferred/absorbed/released/enthalpy/potential energy change when 1 mol/molar amounts of reactant(s) react (to form products) / OWTTE;
under standard conditions / at a pressure 100 kPa/101.3 kPa/1 atm and temperature 298 K/25 °C;
Award [2] for difference between standard enthalpies of products and standard enthalpies of reactants / \({H^\Theta }\) (products) – \({H^\Theta }\) (reactants).
Award [2] for difference between standard enthalpies of formation of products and standard enthalpies of formation of reactants / \(\Sigma \Delta H_f^\Theta \) (products) – \(\Sigma \Delta H_f^\Theta \) (reactants).
(ii) \((1.00 \times 0.0500 = ){\text{ }}0.0500{\text{ (mol)}}\);
\((0.0500 \times 57.9 = ){\text{ }}2.90{\text{ (kJ)}}\);
Ignore any negative sign.
Award [2] for correct final answer.
Award [1 max] for 2900 J.
(iii) \(\left( {\frac{{2.50}}{{40.00}} = } \right){\text{ }}0.0625{\text{ (mol NaOH)}}\);
\(0.0500 \times 4.18 \times 13.3 = 2.78{\text{ (kJ)}}/50.0 \times 4.18 \times 13.3 = 2780{\text{ (J)}}\);
\(\left( {\frac{{2.78}}{{0.0625}}} \right) = - 44.5{\text{ (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}})\);
Award [3] for correct final answer.
Negative sign is necessary for M3.
Award M2 and M3 if is used to obtain an enthalpy change of –46.7 (kJ mol–1).
(iv) \( - 44.5 - 57.9\) / correct Hess’s Law cycle (as below) / correct manipulation of equations;
\( - 102.4{\text{ kJ}}\);
Award [2] for correct final answer.
(i) zinc (only) forms the ion \({\text{Z}}{{\text{n}}^{2 + }}\) / has the oxidation state \( + 2\);
Allow forms only one ion / has only one oxidation state.
has full d-subshell/orbitals / does not have a partially filled d-subshell/orbitals (needed to exhibit transition metal properties);
(ii) \({\text{F}}{{\text{e}}^{2 + }}{\text{: 1}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{p}}^{\text{6}}}{\text{3}}{{\text{s}}^{\text{2}}}{\text{3}}{{\text{p}}^{\text{6}}}{\text{3}}{{\text{d}}^{\text{6}}}/{\text{[Ar] 3}}{{\text{d}}^{\text{6}}}\) and \({\text{F}}{{\text{e}}^{3 + }}{\text{: 1}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{p}}^{\text{6}}}{\text{3}}{{\text{s}}^{\text{2}}}{\text{3}}{{\text{p}}^{\text{6}}}{\text{3}}{{\text{d}}^{\text{5}}}/{\text{[Ar] 3}}{{\text{d}}^{\text{5}}}\);
half-full sub-level/3d5 has extra stability;
less repulsion between electrons / electrons singly occupy orbitals / electrons do not have to pair with other electrons;
Accept converse points for Fe2+.
Examiners report
Most candidates related the rate of reaction to the gradient of the curve, but only a few suggested drawing a tangent at \(t = 0\).
Answers were often disappointing and only a few candidates gained full marks.
Candidates often talked about the number of reactant molecules decreasing but neglected to relate this to a lower concentration. Also some candidates still fail to highlight frequency rather than the number of collisions.
Well answered by more than half of the candidates. The labelling of the axes was a challenge for some candidates. The annotation of the diagram with the energy of activation with and without a catalyst was mostly correct, though some weaker students confused it with the effect of temperature and constructed a second curve. Some candidates could not offer an explanation for the third mark.
(i) Only a few candidates scored this mark. Many candidates stated that a reactant concentration having no effect indicated that the reaction that was zero order in that species, rather than describing the underlying mechanistic reason for the zero order dependence.
(ii) More than half of the candidates could construct a correct rate expression from information about the order of the reactants.
A number of candidates gave a linear relationship, rather than an exponential one, between reaction rate and temperature.
(i) Defining the standard enthalpy change of reaction was not well answered.
(ii) More than half of the candidates calculated the amount of energy released correctly.
(iii) Half of the candidates were able to gain the three marks. Many candidates lost the third mark for not quoting the negative sign for the enthalpy change. Quite a few candidates used a wrong value for the mass of water.
(iv) Many good answers. A Hess’s Law cycle wasn’t often seen. Quite a few candidates scored through ECF from (iii).
(i) Most candidates knew that zinc has a full 3d sub-shell but almost all missed out on the second mark about only having one possible oxidation state in its compounds.
(ii) This was a challenging question for many candidates. A large number of candidates did not give the correct electron configurations for the ions, and only few mentioned the stability of the half-full d-sub-shell. Very few scored the third mark.
When nitrogen gas and hydrogen gas are allowed to react in a closed container the following equilibrium is established.
\[{{\text{N}}_{\text{2}}}{\text{(g)}} + {\text{3}}{{\text{H}}_{\text{2}}}{\text{(g)}} \rightleftharpoons {\text{2N}}{{\text{H}}_{\text{3}}}{\text{(g) }}\Delta H = -92.6{\text{ kJ}}\]
Outline two characteristics of a reversible reaction in a state of dynamic equilibrium.
Predict, with a reason, how each of the following changes affects the position of equilibrium.
The volume of the container is increased.
Ammonia is removed from the equilibrium mixture.
Define the term activation energy, \({E_{\text{a}}}\).
Ammonia is manufactured by the Haber process in which iron is used as a catalyst.
Explain the effect of a catalyst on the rate of reaction.
Typical conditions used in the Haber process are 500 °C and 200 atm, resulting in approximately 15% yield of ammonia.
(i) Explain why a temperature lower than 500 °C is not used.
(ii) Outline why a pressure higher than 200 atm is not often used.
Deduce the equilibrium constant expression, \({K_{\text{c}}}\), for the reaction on page 10.
When 1.00 mol of nitrogen and 3.00 mol of hydrogen were allowed to reach equilibrium in a \({\text{1.00 d}}{{\text{m}}^{\text{3}}}\) container at a temperature of 500 °C and a pressure of 1000 atm, the equilibrium mixture contained 1.46 mol of ammonia.
Calculate the value of \({K_{\text{c}}}\) at 500 °C.
Define the term base according to the Lewis theory.
Define the term weak base according to the Brønsted–Lowry theory.
Deduce the formulas of conjugate acid-base pairs in the reaction below.
\[{\text{C}}{{\text{H}}_{\text{3}}}{\text{N}}{{\text{H}}_{\text{2}}}{\text{(aq)}} + {{\text{H}}_{\text{2}}}{\text{O(l)}} \rightleftharpoons {\text{C}}{{\text{H}}_{\text{3}}}{\text{NH}}_{\text{3}}^ + {\text{(aq)}} + {\text{O}}{{\text{H}}^ - }{\text{(aq)}}\]
Determine the pH of a \({\text{0.100 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) solution of ammonia, \({\text{N}}{{\text{H}}_{\text{3}}}{\text{(aq)}}\), using tables 2 and 15 of the data booklet.
(i) Sketch the pH titration curve obtained when \({\text{50.0 c}}{{\text{m}}^{\text{3}}}\) of \({\text{0.100 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{N}}{{\text{H}}_{\text{3}}}{\text{(aq)}}\) is added to \({\text{25.0 c}}{{\text{m}}^{\text{3}}}\) of \({\text{0.100 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{HCl (aq)}}\).
(ii) Identify an indicator from table 16 of the data booklet that could be used for this titration.
Markscheme
rates of forward and reverse reactions are equal / opposing changes occur at equal rates;
the concentrations of all reactants and products remain constant / macroscopic properties remain constant;
closed/isolated system;
Accept “the same” for “equal” in M1 and for “constant” in M2.
The volume of the container is increased:
position of equilibrium shifts to the left/reactants and fewer moles of gas on the right hand side/pressure decreases / OWTTE;
Ammonia is removed from the equilibrium mixture:
position of equilibrium shifts to the right/products and \({\text{[N}}{{\text{H}}_{\text{3}}}{\text{]}}\) decreases so \({\text{[}}{{\text{N}}_{\text{2}}}{\text{]}}\) and \({\text{[}}{{\text{H}}_{\text{2}}}{\text{]}}\) must also decrease to keep Kc constant
OR
position of equilibrium shifts to the right/products and rate of reverse reaction decreases / OWTTE;
Award [1 max] if both predicted changes are correct.
Do not accept “to increase \([N{H_3}]\)” or reference to LCP without explanation.
minimum energy needed (by reactants/colliding particles) to react/start/initiate a reaction;
Accept “energy difference between reactants and transition state”.
more effective/successful collisions per unit time / greater proportion of collisions effective;
alternative pathway and a lower activation energy
OR
lowers activation energy so that more particles have enough energy to react;
Do not accept just “lowers/reduces the activation energy”.
Accept “provides a surface for reacting/reactants/reaction”.
(i) slower rate / OWTTE;
uneconomic / OWTTE;
(ii) high cost for building/maintaining plant / high energy cost of compressor / OWTTE;
Do not accept “high pressure is expensive” without justification.
Accept high pressure requires high energy.
\(({K_{\text{c}}} = )\frac{{{{{\text{[N}}{{\text{H}}_3}{\text{(g)]}}}^2}}}{{{\text{[}}{{\text{N}}_2}{\text{(g)]}} \times {{{\text{[}}{{\text{H}}_2}{\text{(g)]}}}^3}}}\);
Ignore state symbols.
Concentrations must be represented by square brackets.
moles at equilibrium: nitrogen 0.27, hydrogen 0.81 / concentrations at equilibrium: nitrogen \({\text{0.27 (mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{)}}\), hydrogen \({\text{0.81 (mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{)}}\) (and ammonia \({\text{1.46 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\));
\({K_{\text{c}}} = 15\);
Actual calculation gives \({K_{\text{c}}}{\text{ = }}14{\text{.}}86\).
Award [2] for correct final answer.
Award [1 max] if \({K_{\text{c}}}\left( { = \frac{{{{1.46}^2}}}{{{3^3} \times 1}}} \right) = 0.079\)
electron pair donor;
Accept lone pair donor.
proton acceptor and partially/slightly ionized;
Accept “proton acceptor and partially/slightly dissociated”.
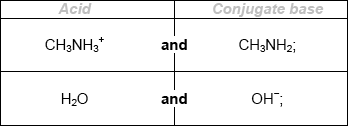
Award [1 max] for two correct acids OR two correct conjugate bases.
\({K_{\text{b}}} = \frac{{{\text{[NH}}_4^ + {\text{][O}}{{\text{H}}^ - }{\text{]}}}}{{{\text{[N}}{{\text{H}}_3}{\text{]}}}} = 1.8 \times {10^{ - 5}}/{10^{ - 4.75}}\);
\({\text{[NH}}_4^ + {\text{]}} = {\text{[O}}{{\text{H}}^ - }{\text{]}}\) and \({\text{[N}}{{\text{H}}_3}{\text{]}} \approx 1.00 \times {10^{ - 1}}{\text{ (mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{)}}\);
\({\text{[O}}{{\text{H}}^ - }{\text{]}} = (\sqrt {1.8 \times {{10}^{ - 6}}} = )1.3 \times {10^{ - 3}}{\text{ (mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{)}}/{\text{pOH}} = 2.89\);
\({\text{pH}} = (14.0 - 2.89 = )11.1\);
Award [4] for correct final answer.
(i) 
For volume \( = 0:{\text{ pH}} = 1\);
vertical jump should be positioned in volume range \({\text{24 c}}{{\text{m}}^{\text{3}}}\) to \({\text{26 c}}{{\text{m}}^{\text{3}}}\) and include pH range between 3 to 6;
For volume = 50: pH between 8 to 11;
(ii) methyl orange / bromophenol blue / bromocresol green / methyl red;
Examiners report
Most candidates were able to give two characteristics of a dynamic equilibrium and explain the effect of changes in volume on the position of equilibrium but many had difficulty giving a complete explanation of the equilibrium shift resulting from the removal of ammonia. Candidates were expected to include a reference to the value of \({K_{\text{c}}}\) or the reduced rate of the reverse reaction when justifying their answer. The definition of activation energy was well known but some lost a mark in their explanation of catalyst action as they did not refer to an alternative pathway in their explanation for the lower activation energy. The explanation of why lower temperatures were not used in the Haber process was also incomplete with many not considering the economic disadvantages of a slow reaction rate. Similarly many did not explain why high pressure was expensive in terms of energy or building costs. Most were able to deduce the equilibrium constant but many lost a mark in the calculation of \({K_{\text{c}}}\) as they used the initial concentrations of nitrogen and hydrogen. Some teachers identified an inconsistency in the question in that the total number of moles of gas under the conditions stated in the question was not consistent with the ideal gas equation however this did not appear to be a problem for the candidates. (However, the ideal gas law cannot be applied here as under these conditions ammonia would be in its supercritical state.) Most candidates were able to define Lewis bases but the definition of weak Brønsted-Lowry bases proved to be more problematic as many did not refer to partial ionisation in their response. Most students were able to identify the conjugate acid-base pairs. The calculation of the pH of an ammonia solution proved to be challenging with many confusing \({K_{\text{a}}}\) and \({K_{\text{b}}}\). Others did not recognize that since it is a weak base, \({\text{[N}}{{\text{H}}_{\text{3}}}{\text{]}}\) at equilibrium is approximately equal to starting concentration \({\text{(0.100 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{)}}\) or that \({\text{[NH}}{{\text{4}}^ + }{\text{]}} = {\text{[O}}{{\text{H}}^ - }{\text{]}}\). (The examination paper was rescaled for candidates sitting the examination in Spanish (due to the error in the question) and candidates close to a boundary given particular attention.) Only the strongest candidates were able to gain full marks for the pH curve although many recognised that the pH would be 1 before any ammonia was added given that HCl is a strong acid. A significant number had the final pH above 11 and did not allow for dilution of the \({\text{0.1 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) ammonia solution. Many correctly identified a possible indicator.
Most candidates were able to give two characteristics of a dynamic equilibrium and explain the effect of changes in volume on the position of equilibrium but many had difficulty giving a complete explanation of the equilibrium shift resulting from the removal of ammonia. Candidates were expected to include a reference to the value of \({K_{\text{c}}}\) or the reduced rate of the reverse reaction when justifying their answer. The definition of activation energy was well known but some lost a mark in their explanation of catalyst action as they did not refer to an alternative pathway in their explanation for the lower activation energy. The explanation of why lower temperatures were not used in the Haber process was also incomplete with many not considering the economic disadvantages of a slow reaction rate. Similarly many did not explain why high pressure was expensive in terms of energy or building costs. Most were able to deduce the equilibrium constant but many lost a mark in the calculation of \({K_{\text{c}}}\) as they used the initial concentrations of nitrogen and hydrogen. Some teachers identified an inconsistency in the question in that the total number of moles of gas under the conditions stated in the question was not consistent with the ideal gas equation however this did not appear to be a problem for the candidates. (However, the ideal gas law cannot be applied here as under these conditions ammonia would be in its supercritical state.) Most candidates were able to define Lewis bases but the definition of weak Brønsted-Lowry bases proved to be more problematic as many did not refer to partial ionisation in their response. Most students were able to identify the conjugate acid-base pairs. The calculation of the pH of an ammonia solution proved to be challenging with many confusing \({K_{\text{a}}}\) and \({K_{\text{b}}}\). Others did not recognize that since it is a weak base, \({\text{[N}}{{\text{H}}_{\text{3}}}{\text{]}}\) at equilibrium is approximately equal to starting concentration \({\text{(0.100 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{)}}\) or that \({\text{[NH}}{{\text{4}}^ + }{\text{]}} = {\text{[O}}{{\text{H}}^ - }{\text{]}}\). (The examination paper was rescaled for candidates sitting the examination in Spanish (due to the error in the question) and candidates close to a boundary given particular attention.) Only the strongest candidates were able to gain full marks for the pH curve although many recognised that the pH would be 1 before any ammonia was added given that HCl is a strong acid. A significant number had the final pH above 11 and did not allow for dilution of the \({\text{0.1 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) ammonia solution. Many correctly identified a possible indicator.
Most candidates were able to give two characteristics of a dynamic equilibrium and explain the effect of changes in volume on the position of equilibrium but many had difficulty giving a complete explanation of the equilibrium shift resulting from the removal of ammonia. Candidates were expected to include a reference to the value of \({K_{\text{c}}}\) or the reduced rate of the reverse reaction when justifying their answer. The definition of activation energy was well known but some lost a mark in their explanation of catalyst action as they did not refer to an alternative pathway in their explanation for the lower activation energy. The explanation of why lower temperatures were not used in the Haber process was also incomplete with many not considering the economic disadvantages of a slow reaction rate. Similarly many did not explain why high pressure was expensive in terms of energy or building costs. Most were able to deduce the equilibrium constant but many lost a mark in the calculation of \({K_{\text{c}}}\) as they used the initial concentrations of nitrogen and hydrogen. Some teachers identified an inconsistency in the question in that the total number of moles of gas under the conditions stated in the question was not consistent with the ideal gas equation however this did not appear to be a problem for the candidates. (However, the ideal gas law cannot be applied here as under these conditions ammonia would be in its supercritical state.) Most candidates were able to define Lewis bases but the definition of weak Brønsted-Lowry bases proved to be more problematic as many did not refer to partial ionisation in their response. Most students were able to identify the conjugate acid-base pairs. The calculation of the pH of an ammonia solution proved to be challenging with many confusing \({K_{\text{a}}}\) and \({K_{\text{b}}}\). Others did not recognize that since it is a weak base, \({\text{[N}}{{\text{H}}_{\text{3}}}{\text{]}}\) at equilibrium is approximately equal to starting concentration \({\text{(0.100 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{)}}\) or that \({\text{[NH}}{{\text{4}}^ + }{\text{]}} = {\text{[O}}{{\text{H}}^ - }{\text{]}}\). (The examination paper was rescaled for candidates sitting the examination in Spanish (due to the error in the question) and candidates close to a boundary given particular attention.) Only the strongest candidates were able to gain full marks for the pH curve although many recognised that the pH would be 1 before any ammonia was added given that HCl is a strong acid. A significant number had the final pH above 11 and did not allow for dilution of the \({\text{0.1 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) ammonia solution. Many correctly identified a possible indicator.
Most candidates were able to give two characteristics of a dynamic equilibrium and explain the effect of changes in volume on the position of equilibrium but many had difficulty giving a complete explanation of the equilibrium shift resulting from the removal of ammonia. Candidates were expected to include a reference to the value of \({K_{\text{c}}}\) or the reduced rate of the reverse reaction when justifying their answer. The definition of activation energy was well known but some lost a mark in their explanation of catalyst action as they did not refer to an alternative pathway in their explanation for the lower activation energy. The explanation of why lower temperatures were not used in the Haber process was also incomplete with many not considering the economic disadvantages of a slow reaction rate. Similarly many did not explain why high pressure was expensive in terms of energy or building costs. Most were able to deduce the equilibrium constant but many lost a mark in the calculation of \({K_{\text{c}}}\) as they used the initial concentrations of nitrogen and hydrogen. Some teachers identified an inconsistency in the question in that the total number of moles of gas under the conditions stated in the question was not consistent with the ideal gas equation however this did not appear to be a problem for the candidates. (However, the ideal gas law cannot be applied here as under these conditions ammonia would be in its supercritical state.) Most candidates were able to define Lewis bases but the definition of weak Brønsted-Lowry bases proved to be more problematic as many did not refer to partial ionisation in their response. Most students were able to identify the conjugate acid-base pairs. The calculation of the pH of an ammonia solution proved to be challenging with many confusing \({K_{\text{a}}}\) and \({K_{\text{b}}}\). Others did not recognize that since it is a weak base, \({\text{[N}}{{\text{H}}_{\text{3}}}{\text{]}}\) at equilibrium is approximately equal to starting concentration \({\text{(0.100 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{)}}\) or that \({\text{[NH}}{{\text{4}}^ + }{\text{]}} = {\text{[O}}{{\text{H}}^ - }{\text{]}}\). (The examination paper was rescaled for candidates sitting the examination in Spanish (due to the error in the question) and candidates close to a boundary given particular attention.) Only the strongest candidates were able to gain full marks for the pH curve although many recognised that the pH would be 1 before any ammonia was added given that HCl is a strong acid. A significant number had the final pH above 11 and did not allow for dilution of the \({\text{0.1 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) ammonia solution. Many correctly identified a possible indicator.
Most candidates were able to give two characteristics of a dynamic equilibrium and explain the effect of changes in volume on the position of equilibrium but many had difficulty giving a complete explanation of the equilibrium shift resulting from the removal of ammonia. Candidates were expected to include a reference to the value of \({K_{\text{c}}}\) or the reduced rate of the reverse reaction when justifying their answer. The definition of activation energy was well known but some lost a mark in their explanation of catalyst action as they did not refer to an alternative pathway in their explanation for the lower activation energy. The explanation of why lower temperatures were not used in the Haber process was also incomplete with many not considering the economic disadvantages of a slow reaction rate. Similarly many did not explain why high pressure was expensive in terms of energy or building costs. Most were able to deduce the equilibrium constant but many lost a mark in the calculation of \({K_{\text{c}}}\) as they used the initial concentrations of nitrogen and hydrogen. Some teachers identified an inconsistency in the question in that the total number of moles of gas under the conditions stated in the question was not consistent with the ideal gas equation however this did not appear to be a problem for the candidates. (However, the ideal gas law cannot be applied here as under these conditions ammonia would be in its supercritical state.) Most candidates were able to define Lewis bases but the definition of weak Brønsted-Lowry bases proved to be more problematic as many did not refer to partial ionisation in their response. Most students were able to identify the conjugate acid-base pairs. The calculation of the pH of an ammonia solution proved to be challenging with many confusing \({K_{\text{a}}}\) and \({K_{\text{b}}}\). Others did not recognize that since it is a weak base, \({\text{[N}}{{\text{H}}_{\text{3}}}{\text{]}}\) at equilibrium is approximately equal to starting concentration \({\text{(0.100 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{)}}\) or that \({\text{[NH}}{{\text{4}}^ + }{\text{]}} = {\text{[O}}{{\text{H}}^ - }{\text{]}}\). (The examination paper was rescaled for candidates sitting the examination in Spanish (due to the error in the question) and candidates close to a boundary given particular attention.) Only the strongest candidates were able to gain full marks for the pH curve although many recognised that the pH would be 1 before any ammonia was added given that HCl is a strong acid. A significant number had the final pH above 11 and did not allow for dilution of the \({\text{0.1 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) ammonia solution. Many correctly identified a possible indicator.
Most candidates were able to give two characteristics of a dynamic equilibrium and explain the effect of changes in volume on the position of equilibrium but many had difficulty giving a complete explanation of the equilibrium shift resulting from the removal of ammonia. Candidates were expected to include a reference to the value of \({K_{\text{c}}}\) or the reduced rate of the reverse reaction when justifying their answer. The definition of activation energy was well known but some lost a mark in their explanation of catalyst action as they did not refer to an alternative pathway in their explanation for the lower activation energy. The explanation of why lower temperatures were not used in the Haber process was also incomplete with many not considering the economic disadvantages of a slow reaction rate. Similarly many did not explain why high pressure was expensive in terms of energy or building costs. Most were able to deduce the equilibrium constant but many lost a mark in the calculation of \({K_{\text{c}}}\) as they used the initial concentrations of nitrogen and hydrogen. Some teachers identified an inconsistency in the question in that the total number of moles of gas under the conditions stated in the question was not consistent with the ideal gas equation however this did not appear to be a problem for the candidates. (However, the ideal gas law cannot be applied here as under these conditions ammonia would be in its supercritical state.) Most candidates were able to define Lewis bases but the definition of weak Brønsted-Lowry bases proved to be more problematic as many did not refer to partial ionisation in their response. Most students were able to identify the conjugate acid-base pairs. The calculation of the pH of an ammonia solution proved to be challenging with many confusing \({K_{\text{a}}}\) and \({K_{\text{b}}}\). Others did not recognize that since it is a weak base, \({\text{[N}}{{\text{H}}_{\text{3}}}{\text{]}}\) at equilibrium is approximately equal to starting concentration \({\text{(0.100 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{)}}\) or that \({\text{[NH}}{{\text{4}}^ + }{\text{]}} = {\text{[O}}{{\text{H}}^ - }{\text{]}}\). (The examination paper was rescaled for candidates sitting the examination in Spanish (due to the error in the question) and candidates close to a boundary given particular attention.) Only the strongest candidates were able to gain full marks for the pH curve although many recognised that the pH would be 1 before any ammonia was added given that HCl is a strong acid. A significant number had the final pH above 11 and did not allow for dilution of the \({\text{0.1 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) ammonia solution. Many correctly identified a possible indicator.
Most candidates were able to give two characteristics of a dynamic equilibrium and explain the effect of changes in volume on the position of equilibrium but many had difficulty giving a complete explanation of the equilibrium shift resulting from the removal of ammonia. Candidates were expected to include a reference to the value of \({K_{\text{c}}}\) or the reduced rate of the reverse reaction when justifying their answer. The definition of activation energy was well known but some lost a mark in their explanation of catalyst action as they did not refer to an alternative pathway in their explanation for the lower activation energy. The explanation of why lower temperatures were not used in the Haber process was also incomplete with many not considering the economic disadvantages of a slow reaction rate. Similarly many did not explain why high pressure was expensive in terms of energy or building costs. Most were able to deduce the equilibrium constant but many lost a mark in the calculation of \({K_{\text{c}}}\) as they used the initial concentrations of nitrogen and hydrogen. Some teachers identified an inconsistency in the question in that the total number of moles of gas under the conditions stated in the question was not consistent with the ideal gas equation however this did not appear to be a problem for the candidates. (However, the ideal gas law cannot be applied here as under these conditions ammonia would be in its supercritical state.) Most candidates were able to define Lewis bases but the definition of weak Brønsted-Lowry bases proved to be more problematic as many did not refer to partial ionisation in their response. Most students were able to identify the conjugate acid-base pairs. The calculation of the pH of an ammonia solution proved to be challenging with many confusing \({K_{\text{a}}}\) and \({K_{\text{b}}}\). Others did not recognize that since it is a weak base, \({\text{[N}}{{\text{H}}_{\text{3}}}{\text{]}}\) at equilibrium is approximately equal to starting concentration \({\text{(0.100 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{)}}\) or that \({\text{[NH}}{{\text{4}}^ + }{\text{]}} = {\text{[O}}{{\text{H}}^ - }{\text{]}}\). (The examination paper was rescaled for candidates sitting the examination in Spanish (due to the error in the question) and candidates close to a boundary given particular attention.) Only the strongest candidates were able to gain full marks for the pH curve although many recognised that the pH would be 1 before any ammonia was added given that HCl is a strong acid. A significant number had the final pH above 11 and did not allow for dilution of the \({\text{0.1 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) ammonia solution. Many correctly identified a possible indicator.
Most candidates were able to give two characteristics of a dynamic equilibrium and explain the effect of changes in volume on the position of equilibrium but many had difficulty giving a complete explanation of the equilibrium shift resulting from the removal of ammonia. Candidates were expected to include a reference to the value of \({K_{\text{c}}}\) or the reduced rate of the reverse reaction when justifying their answer. The definition of activation energy was well known but some lost a mark in their explanation of catalyst action as they did not refer to an alternative pathway in their explanation for the lower activation energy. The explanation of why lower temperatures were not used in the Haber process was also incomplete with many not considering the economic disadvantages of a slow reaction rate. Similarly many did not explain why high pressure was expensive in terms of energy or building costs. Most were able to deduce the equilibrium constant but many lost a mark in the calculation of \({K_{\text{c}}}\) as they used the initial concentrations of nitrogen and hydrogen. Some teachers identified an inconsistency in the question in that the total number of moles of gas under the conditions stated in the question was not consistent with the ideal gas equation however this did not appear to be a problem for the candidates. (However, the ideal gas law cannot be applied here as under these conditions ammonia would be in its supercritical state.) Most candidates were able to define Lewis bases but the definition of weak Brønsted-Lowry bases proved to be more problematic as many did not refer to partial ionisation in their response. Most students were able to identify the conjugate acid-base pairs. The calculation of the pH of an ammonia solution proved to be challenging with many confusing \({K_{\text{a}}}\) and \({K_{\text{b}}}\). Others did not recognize that since it is a weak base, \({\text{[N}}{{\text{H}}_{\text{3}}}{\text{]}}\) at equilibrium is approximately equal to starting concentration \({\text{(0.100 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{)}}\) or that \({\text{[NH}}{{\text{4}}^ + }{\text{]}} = {\text{[O}}{{\text{H}}^ - }{\text{]}}\). (The examination paper was rescaled for candidates sitting the examination in Spanish (due to the error in the question) and candidates close to a boundary given particular attention.) Only the strongest candidates were able to gain full marks for the pH curve although many recognised that the pH would be 1 before any ammonia was added given that HCl is a strong acid. A significant number had the final pH above 11 and did not allow for dilution of the \({\text{0.1 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) ammonia solution. Many correctly identified a possible indicator.
Most candidates were able to give two characteristics of a dynamic equilibrium and explain the effect of changes in volume on the position of equilibrium but many had difficulty giving a complete explanation of the equilibrium shift resulting from the removal of ammonia. Candidates were expected to include a reference to the value of \({K_{\text{c}}}\) or the reduced rate of the reverse reaction when justifying their answer. The definition of activation energy was well known but some lost a mark in their explanation of catalyst action as they did not refer to an alternative pathway in their explanation for the lower activation energy. The explanation of why lower temperatures were not used in the Haber process was also incomplete with many not considering the economic disadvantages of a slow reaction rate. Similarly many did not explain why high pressure was expensive in terms of energy or building costs. Most were able to deduce the equilibrium constant but many lost a mark in the calculation of \({K_{\text{c}}}\) as they used the initial concentrations of nitrogen and hydrogen. Some teachers identified an inconsistency in the question in that the total number of moles of gas under the conditions stated in the question was not consistent with the ideal gas equation however this did not appear to be a problem for the candidates. (However, the ideal gas law cannot be applied here as under these conditions ammonia would be in its supercritical state.) Most candidates were able to define Lewis bases but the definition of weak Brønsted-Lowry bases proved to be more problematic as many did not refer to partial ionisation in their response. Most students were able to identify the conjugate acid-base pairs. The calculation of the pH of an ammonia solution proved to be challenging with many confusing \({K_{\text{a}}}\) and \({K_{\text{b}}}\). Others did not recognize that since it is a weak base, \({\text{[N}}{{\text{H}}_{\text{3}}}{\text{]}}\) at equilibrium is approximately equal to starting concentration \({\text{(0.100 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{)}}\) or that \({\text{[NH}}{{\text{4}}^ + }{\text{]}} = {\text{[O}}{{\text{H}}^ - }{\text{]}}\). (The examination paper was rescaled for candidates sitting the examination in Spanish (due to the error in the question) and candidates close to a boundary given particular attention.) Only the strongest candidates were able to gain full marks for the pH curve although many recognised that the pH would be 1 before any ammonia was added given that HCl is a strong acid. A significant number had the final pH above 11 and did not allow for dilution of the \({\text{0.1 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) ammonia solution. Many correctly identified a possible indicator.
Most candidates were able to give two characteristics of a dynamic equilibrium and explain the effect of changes in volume on the position of equilibrium but many had difficulty giving a complete explanation of the equilibrium shift resulting from the removal of ammonia. Candidates were expected to include a reference to the value of \({K_{\text{c}}}\) or the reduced rate of the reverse reaction when justifying their answer. The definition of activation energy was well known but some lost a mark in their explanation of catalyst action as they did not refer to an alternative pathway in their explanation for the lower activation energy. The explanation of why lower temperatures were not used in the Haber process was also incomplete with many not considering the economic disadvantages of a slow reaction rate. Similarly many did not explain why high pressure was expensive in terms of energy or building costs. Most were able to deduce the equilibrium constant but many lost a mark in the calculation of \({K_{\text{c}}}\) as they used the initial concentrations of nitrogen and hydrogen. Some teachers identified an inconsistency in the question in that the total number of moles of gas under the conditions stated in the question was not consistent with the ideal gas equation however this did not appear to be a problem for the candidates. (However, the ideal gas law cannot be applied here as under these conditions ammonia would be in its supercritical state.) Most candidates were able to define Lewis bases but the definition of weak Brønsted-Lowry bases proved to be more problematic as many did not refer to partial ionisation in their response. Most students were able to identify the conjugate acid-base pairs. The calculation of the pH of an ammonia solution proved to be challenging with many confusing \({K_{\text{a}}}\) and \({K_{\text{b}}}\). Others did not recognize that since it is a weak base, \({\text{[N}}{{\text{H}}_{\text{3}}}{\text{]}}\) at equilibrium is approximately equal to starting concentration \({\text{(0.100 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{)}}\) or that \({\text{[NH}}{{\text{4}}^ + }{\text{]}} = {\text{[O}}{{\text{H}}^ - }{\text{]}}\). (The examination paper was rescaled for candidates sitting the examination in Spanish (due to the error in the question) and candidates close to a boundary given particular attention.) Only the strongest candidates were able to gain full marks for the pH curve although many recognised that the pH would be 1 before any ammonia was added given that HCl is a strong acid. A significant number had the final pH above 11 and did not allow for dilution of the \({\text{0.1 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) ammonia solution. Many correctly identified a possible indicator.
Most candidates were able to give two characteristics of a dynamic equilibrium and explain the effect of changes in volume on the position of equilibrium but many had difficulty giving a complete explanation of the equilibrium shift resulting from the removal of ammonia. Candidates were expected to include a reference to the value of \({K_{\text{c}}}\) or the reduced rate of the reverse reaction when justifying their answer. The definition of activation energy was well known but some lost a mark in their explanation of catalyst action as they did not refer to an alternative pathway in their explanation for the lower activation energy. The explanation of why lower temperatures were not used in the Haber process was also incomplete with many not considering the economic disadvantages of a slow reaction rate. Similarly many did not explain why high pressure was expensive in terms of energy or building costs. Most were able to deduce the equilibrium constant but many lost a mark in the calculation of \({K_{\text{c}}}\) as they used the initial concentrations of nitrogen and hydrogen. Some teachers identified an inconsistency in the question in that the total number of moles of gas under the conditions stated in the question was not consistent with the ideal gas equation however this did not appear to be a problem for the candidates. (However, the ideal gas law cannot be applied here as under these conditions ammonia would be in its supercritical state.) Most candidates were able to define Lewis bases but the definition of weak Brønsted-Lowry bases proved to be more problematic as many did not refer to partial ionisation in their response. Most students were able to identify the conjugate acid-base pairs. The calculation of the pH of an ammonia solution proved to be challenging with many confusing \({K_{\text{a}}}\) and \({K_{\text{b}}}\). Others did not recognize that since it is a weak base, \({\text{[N}}{{\text{H}}_{\text{3}}}{\text{]}}\) at equilibrium is approximately equal to starting concentration \({\text{(0.100 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{)}}\) or that \({\text{[NH}}{{\text{4}}^ + }{\text{]}} = {\text{[O}}{{\text{H}}^ - }{\text{]}}\). (The examination paper was rescaled for candidates sitting the examination in Spanish (due to the error in the question) and candidates close to a boundary given particular attention.) Only the strongest candidates were able to gain full marks for the pH curve although many recognised that the pH would be 1 before any ammonia was added given that HCl is a strong acid. A significant number had the final pH above 11 and did not allow for dilution of the \({\text{0.1 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) ammonia solution. Many correctly identified a possible indicator.
Most candidates were able to give two characteristics of a dynamic equilibrium and explain the effect of changes in volume on the position of equilibrium but many had difficulty giving a complete explanation of the equilibrium shift resulting from the removal of ammonia. Candidates were expected to include a reference to the value of \({K_{\text{c}}}\) or the reduced rate of the reverse reaction when justifying their answer. The definition of activation energy was well known but some lost a mark in their explanation of catalyst action as they did not refer to an alternative pathway in their explanation for the lower activation energy. The explanation of why lower temperatures were not used in the Haber process was also incomplete with many not considering the economic disadvantages of a slow reaction rate. Similarly many did not explain why high pressure was expensive in terms of energy or building costs. Most were able to deduce the equilibrium constant but many lost a mark in the calculation of \({K_{\text{c}}}\) as they used the initial concentrations of nitrogen and hydrogen. Some teachers identified an inconsistency in the question in that the total number of moles of gas under the conditions stated in the question was not consistent with the ideal gas equation however this did not appear to be a problem for the candidates. (However, the ideal gas law cannot be applied here as under these conditions ammonia would be in its supercritical state.) Most candidates were able to define Lewis bases but the definition of weak Brønsted-Lowry bases proved to be more problematic as many did not refer to partial ionisation in their response. Most students were able to identify the conjugate acid-base pairs. The calculation of the pH of an ammonia solution proved to be challenging with many confusing \({K_{\text{a}}}\) and \({K_{\text{b}}}\). Others did not recognize that since it is a weak base, \({\text{[N}}{{\text{H}}_{\text{3}}}{\text{]}}\) at equilibrium is approximately equal to starting concentration \({\text{(0.100 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{)}}\) or that \({\text{[NH}}{{\text{4}}^ + }{\text{]}} = {\text{[O}}{{\text{H}}^ - }{\text{]}}\). (The examination paper was rescaled for candidates sitting the examination in Spanish (due to the error in the question) and candidates close to a boundary given particular attention.) Only the strongest candidates were able to gain full marks for the pH curve although many recognised that the pH would be 1 before any ammonia was added given that HCl is a strong acid. A significant number had the final pH above 11 and did not allow for dilution of the \({\text{0.1 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) ammonia solution. Many correctly identified a possible indicator.
Nitrogen(II) oxide reacts with hydrogen according to the equation below.
\[{\text{2NO(g)}} + {\text{2}}{{\text{H}}_{\text{2}}}{\text{(g)}} \to {{\text{N}}_{\text{2}}}{\text{(g)}} + {\text{2}}{{\text{H}}_{\text{2}}}{\text{O(g)}}\]
A suggested mechanism for this reaction is:
Step 1: \({\text{NO}} + {{\text{H}}_{\text{2}}} \rightleftharpoons {\text{X}}\) fast
Step 2: \({\text{X}} + {\text{NO}} \to {\text{Y}} + {{\text{H}}_{\text{2}}}{\text{O}}\) slow
Step 3: \({\text{Y}} + {{\text{H}}_{\text{2}}} \to {{\text{N}}_{\text{2}}} + {{\text{H}}_{\text{2}}}{\text{O}}\) fast
Define the term rate of reaction.
Explain why increasing the particle size of a solid reactant decreases the rate of reaction.
Identify the rate-determining step.
A student hypothesized that the order of reaction with respect to \({{\text{H}}_{\text{2}}}\) is 2.
Evaluate this hypothesis.
Markscheme
change in concentration of reactant/product with time / rate of change of concentration;
Accept “increase” instead of “change” for product and “decrease” instead of “change” for reactant.
Accept “mass/amount/volume” instead of “concentration”.
Do not accept substance.
surface area decreases;
frequency/probability of collisions decreases;
Accept number of collisions per unit time decreases.
step 2 \({\text{/ X}} + {\text{NO}} \to {\text{Y}} + {{\text{H}}_{\text{2}}}{\text{O /}}\) slow;
invalid / unlikely as order most likely one (with respect to hydrogen);
\({\text{rate}} = k{{\text{[NO]}}^{\text{2}}}{\text{[}}{{\text{H}}_{\text{2}}}{\text{] / }}{{\text{H}}_{\text{2}}}\) only involved once in the formation of the intermediate before the slow step / OWTTE;
Award M2 only if M1 is correct.
Examiners report
Although most candidates were able to define the rate of reaction, some of weaker candidates gave imprecise answers which did not refer to concentration of the reactants or products and the “the time for the reaction to go to completion” was not an uncommon response. Most candidates realized that the surface area would decrease but, as in previous sessions, lost marks as they did not refer to the reduced “frequency” of collisions. Most candidates were able to identify the rate determining step and correctly state that the reaction would be first order with respect to hydrogen however only a minority could explain their answer in sufficient detail i.e. that \({{\text{H}}_{\text{2}}}\) was involved only once in the formation of the intermediate before the rate determining step.
Although most candidates were able to define the rate of reaction, some of weaker candidates gave imprecise answers which did not refer to concentration of the reactants or products and the “the time for the reaction to go to completion” was not an uncommon response. Most candidates realized that the surface area would decrease but, as in previous sessions, lost marks as they did not refer to the reduced “frequency” of collisions. Most candidates were able to identify the rate determining step and correctly state that the reaction would be first order with respect to hydrogen however only a minority could explain their answer in sufficient detail i.e. that \({{\text{H}}_{\text{2}}}\) was involved only once in the formation of the intermediate before the rate determining step.
Although most candidates were able to define the rate of reaction, some of weaker candidates gave imprecise answers which did not refer to concentration of the reactants or products and the “the time for the reaction to go to completion” was not an uncommon response. Most candidates realized that the surface area would decrease but, as in previous sessions, lost marks as they did not refer to the reduced “frequency” of collisions. Most candidates were able to identify the rate determining step and correctly state that the reaction would be first order with respect to hydrogen however only a minority could explain their answer in sufficient detail i.e. that \({{\text{H}}_{\text{2}}}\) was involved only once in the formation of the intermediate before the rate determining step.
Although most candidates were able to define the rate of reaction, some of weaker candidates gave imprecise answers which did not refer to concentration of the reactants or products and the “the time for the reaction to go to completion” was not an uncommon response. Most candidates realized that the surface area would decrease but, as in previous sessions, lost marks as they did not refer to the reduced “frequency” of collisions. Most candidates were able to identify the rate determining step and correctly state that the reaction would be first order with respect to hydrogen however only a minority could explain their answer in sufficient detail i.e. that \({{\text{H}}_{\text{2}}}\) was involved only once in the formation of the intermediate before the rate determining step.
A class studied the equilibrium established when ethanoic acid and ethanol react together in the presence of a strong acid, using propanone as an inert solvent. The equation is given below.
\[{\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}} + {{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}{\text{OH}} \rightleftharpoons {\text{C}}{{\text{H}}_{\text{3}}}{\text{COO}}{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}} + {{\text{H}}_{\text{2}}}{\text{O}}\]
One group made the following initial mixture:
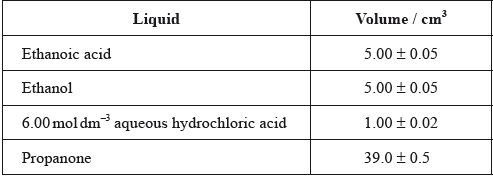
After one week, a \(5.00 \pm 0.05{\text{ c}}{{\text{m}}^{\text{3}}}\) sample of the final equilibrium mixture was pipetted out and titrated with \({\text{0.200 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) aqueous sodium hydroxide to determine the amount of ethanoic acid remaining. The following titration results were obtained:
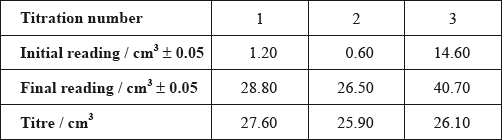
The density of ethanoic acid is \({\text{1.05 g}}\,{\text{c}}{{\text{m}}^{ - 3}}\). Determine the amount, in mol, of ethanoic acid present in the initial mixture.
The concentration of ethanoic acid can be calculated as \({\text{1.748 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\). Determine the percentage uncertainty of this value. (Neglect any uncertainty in the density and the molar mass.)
Calculate the absolute uncertainty of the titre for Titration 1 (\({\text{27.60 c}}{{\text{m}}^3}\)).
Suggest the average volume of alkali, required to neutralize the \({\text{5.00 c}}{{\text{m}}^{\text{3}}}\) sample, that the student should use.
\({\text{3.00 c}}{{\text{m}}^{\text{3}}}\) of the \({\text{0.200 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) aqueous sodium hydroxide reacted with the hydrochloric acid present in the \({\text{5.00 c}}{{\text{m}}^{\text{3}}}\) sample. Determine the concentration of ethanoic acid in the final equilibrium mixture.
Deduce the equilibrium constant expression for the reaction.
The other concentrations in the equilibrium mixture were calculated as follows:
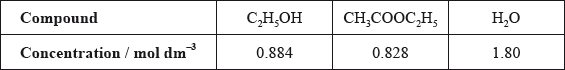
Use these data, along with your answer to part (iii), to determine the value of the equilibrium constant. (If you did not obtain an answer to part (iii), assume the concentrations of ethanol and ethanoic acid are equal, although this is not the case.)
Outline how you could establish that the system had reached equilibrium at the end of one week.
Outline why changing the temperature has only a very small effect on the value of the equilibrium constant for this equilibrium.
Outline how adding some ethyl ethanoate to the initial mixture would affect the amount of ethanoic acid converted to product.
Propanone is used as the solvent because one compound involved in the equilibrium is insoluble in water. Identify this compound and explain why it is insoluble in water.
Suggest one other reason why using water as a solvent would make the experiment less successful.
Markscheme
\({\text{M(C}}{{\text{H}}_{\text{3}}}{\text{COOH)}}\left( { = (4 \times 1.01) + (2 \times 12.01) + (2 \times 16.00)} \right) = 60.06{\text{ (g}}\,{\text{mo}}{{\text{l}}^{ - 1}})\);
Accept 60 (g mol–1).
\({\text{mass (C}}{{\text{H}}_3}{\text{COOH) }}( = 5.00 \times 1.05) = 5.25{\text{ (g)}}\);
\(\frac{{5.25}}{{60.06}} = 0.0874{\text{ (mol)}}\);
Award [3] for correct final answer.
Accept 0.0875 (comes from using Mr = 60 g mol–1).
percentage uncertainty in volume of ethanoic acid \( = 100 \times \frac{{0.05}}{{5.00}}{\text{ }} = 1\% \);
percentage uncertainty in total volume \( = 100 \times \frac{{0.62}}{{50}} = 1.24\% \);
total percentage uncertainty \( = 1 + 1.24 = 2.24\% \);
Accept rounding down to 2.2/2%.
\( \pm 0.1/0.10{\text{ }}({\text{c}}{{\text{m}}^3})\);
Do not accept without ±.
\({\text{26.00 (c}}{{\text{m}}^{\text{3}}}{\text{)}}\);
\(26.00 - 3.00 = 23.00{\text{ }}({\text{c}}{{\text{m}}^3})\);
If other methods used, award M1 for calculating amount of NaOH reacting with CH3COOH.
\(0.200 \times \frac{{23.00}}{{5.00}} = 0.920{\text{ }}({\text{mol}}\,{\text{d}}{{\text{m}}^{ - 3}})\);
Award [2] for correct final answer.
If (ii) given as mean titre (26.5 cm3) then ECF answer comes to 0.94 (mol dm–3).
\(({K_{\text{c}}} = )\frac{{{\text{[C}}{{\text{H}}_3}{\text{COO}}{{\text{C}}_2}{{\text{H}}_5}{\text{][}}{{\text{H}}_2}{\text{O]}}}}{{{\text{[}}{{\text{C}}_2}{{\text{H}}_5}{\text{OH][C}}{{\text{H}}_3}{\text{COOH]}}}}\);
Do not penalize minor errors in formulas.
Accept \(({K_{\text{c}}} = )\frac{{{\text{[}}esther{\text{][}}water{\text{]}}}}{{[ethanol/alcohol{\text{][(}}ethanoic{\text{) }}acid{\text{]}}}}\).
\(({K_c} = )\frac{{0.828 \times 1.80}}{{0.884 \times 0.920}} = 1.83\);
If assumed [CH3COOH] = 0.884 mol dm-3, answer is 1.91 – allow this even if an answer was obtained for (iii).
If (ii) given as mean titre (26.5 cm3) then ECF answer comes to 1.79.
repeat the titration a day/week later (and result should be the same) / OWTTE;
Accept “concentrations/physical properties/macroscopic properties of the system do not change”.
enthalpy change/\(\Delta H\) for the reaction is (very) small / OWTTE;
decreases (the amount of ethanoic acid converted);
Accept “increases amount of ethanoic acid present at equilibrium” / OWTTE.
(adding product) shifts position of equilibrium towards reactants/LHS / increases
the rate of the reverse reaction / OWTTE;
ethyl ethanoate/\({\text{C}}{{\text{H}}_{\text{3}}}{\text{COO}}{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}\)/ester;
forms only weak hydrogen bonds (to water);
Allow “does not hydrogen bond to water” / “hydrocarbon sections too long” / OWTTE.
M2 can only be given only if M1 correct.
(large excess of) water will shift the position of equilibrium (far to the left) / OWTTE;
Accept any other chemically sound response, such as “dissociation of ethanoic acid would affect equilibrium”.
Examiners report
Generally candidates found this question quite challenging and some left quite a number of parts unanswered. The tradition is that the first question on the paper is a data response question, which often addresses many aspects of the syllabus, and unfortunately candidates, especially those of average or below average ability, seem to have difficulty in tackling questions of this nature. One other issue with data response questions is that, of necessity, the data appears at the beginning of the question whilst, mainly because of the space left for candidates to answer, the later parts of the question referring to these data may not appear until a number of pages into the paper.
Part (a) concerning density, volume and amount of substance was generally reasonably well answered, but the following parts, concerning uncertainties, were rarely answered correctly and a number confused precision (uncertainty, either absolute or as a percentage) and accuracy (percentage error in the value obtained). Many candidates also seemed to lack experimental common sense, simply taking an average that included an initial titre that was much larger than the concordant second and third titres, rather than excluding it. This lack of experimental “know how” was also evident in responses to (c) (iii) where it was unusual for the approach to the question to indicate the candidate had realised that the alkali was neutralising two different acids (HCl and \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}\)) and again in part (d) where it was rare for the response to outline a practical solution to the problem, though quite a number of candidates suggested that the pH would become constant, presumably not realising that the pH would be dominated by the HCl catalyst. Most students could however carry out the more routine tasks of writing an equilibrium constant expression and determining its value from the data given. Many candidates were aware of Le Chatelier effects on the position of equilibrium, but a significant number failed to use this information to answer the question actually asked and the unusual approach to the effect of temperature disconcerted many. Whilst most students managed to identify the ester as the component of the mixture that was insoluble in water, the reasons given were usually couched in terms of the polarity of the molecule (many quite polar molecules, halogenoalkanes for example, are insoluble in water) rather than its inability to form strong hydrogen bonds to water, which is the critical factor. Quite a number of students came up with a valid reason why water would not be a suitable solvent, though some students appeared to have overlooked the fact the question stated “other reason”.
Generally candidates found this question quite challenging and some left quite a number of parts unanswered. The tradition is that the first question on the paper is a data response question, which often addresses many aspects of the syllabus, and unfortunately candidates, especially those of average or below average ability, seem to have difficulty in tackling questions of this nature. One other issue with data response questions is that, of necessity, the data appears at the beginning of the question whilst, mainly because of the space left for candidates to answer, the later parts of the question referring to these data may not appear until a number of pages into the paper.
Part (a) concerning density, volume and amount of substance was generally reasonably well answered, but the following parts, concerning uncertainties, were rarely answered correctly and a number confused precision (uncertainty, either absolute or as a percentage) and accuracy (percentage error in the value obtained). Many candidates also seemed to lack experimental common sense, simply taking an average that included an initial titre that was much larger than the concordant second and third titres, rather than excluding it. This lack of experimental “know how” was also evident in responses to (c) (iii) where it was unusual for the approach to the question to indicate the candidate had realised that the alkali was neutralising two different acids (HCl and \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}\)) and again in part (d) where it was rare for the response to outline a practical solution to the problem, though quite a number of candidates suggested that the pH would become constant, presumably not realising that the pH would be dominated by the HCl catalyst. Most students could however carry out the more routine tasks of writing an equilibrium constant expression and determining its value from the data given. Many candidates were aware of Le Chatelier effects on the position of equilibrium, but a significant number failed to use this information to answer the question actually asked and the unusual approach to the effect of temperature disconcerted many. Whilst most students managed to identify the ester as the component of the mixture that was insoluble in water, the reasons given were usually couched in terms of the polarity of the molecule (many quite polar molecules, halogenoalkanes for example, are insoluble in water) rather than its inability to form strong hydrogen bonds to water, which is the critical factor. Quite a number of students came up with a valid reason why water would not be a suitable solvent, though some students appeared to have overlooked the fact the question stated “other reason”.
Generally candidates found this question quite challenging and some left quite a number of parts unanswered. The tradition is that the first question on the paper is a data response question, which often addresses many aspects of the syllabus, and unfortunately candidates, especially those of average or below average ability, seem to have difficulty in tackling questions of this nature. One other issue with data response questions is that, of necessity, the data appears at the beginning of the question whilst, mainly because of the space left for candidates to answer, the later parts of the question referring to these data may not appear until a number of pages into the paper.
Part (a) concerning density, volume and amount of substance was generally reasonably well answered, but the following parts, concerning uncertainties, were rarely answered correctly and a number confused precision (uncertainty, either absolute or as a percentage) and accuracy (percentage error in the value obtained). Many candidates also seemed to lack experimental common sense, simply taking an average that included an initial titre that was much larger than the concordant second and third titres, rather than excluding it. This lack of experimental “know how” was also evident in responses to (c) (iii) where it was unusual for the approach to the question to indicate the candidate had realised that the alkali was neutralising two different acids (HCl and \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}\)) and again in part (d) where it was rare for the response to outline a practical solution to the problem, though quite a number of candidates suggested that the pH would become constant, presumably not realising that the pH would be dominated by the HCl catalyst. Most students could however carry out the more routine tasks of writing an equilibrium constant expression and determining its value from the data given. Many candidates were aware of Le Chatelier effects on the position of equilibrium, but a significant number failed to use this information to answer the question actually asked and the unusual approach to the effect of temperature disconcerted many. Whilst most students managed to identify the ester as the component of the mixture that was insoluble in water, the reasons given were usually couched in terms of the polarity of the molecule (many quite polar molecules, halogenoalkanes for example, are insoluble in water) rather than its inability to form strong hydrogen bonds to water, which is the critical factor. Quite a number of students came up with a valid reason why water would not be a suitable solvent, though some students appeared to have overlooked the fact the question stated “other reason”.
Generally candidates found this question quite challenging and some left quite a number of parts unanswered. The tradition is that the first question on the paper is a data response question, which often addresses many aspects of the syllabus, and unfortunately candidates, especially those of average or below average ability, seem to have difficulty in tackling questions of this nature. One other issue with data response questions is that, of necessity, the data appears at the beginning of the question whilst, mainly because of the space left for candidates to answer, the later parts of the question referring to these data may not appear until a number of pages into the paper.
Part (a) concerning density, volume and amount of substance was generally reasonably well answered, but the following parts, concerning uncertainties, were rarely answered correctly and a number confused precision (uncertainty, either absolute or as a percentage) and accuracy (percentage error in the value obtained). Many candidates also seemed to lack experimental common sense, simply taking an average that included an initial titre that was much larger than the concordant second and third titres, rather than excluding it. This lack of experimental “know how” was also evident in responses to (c) (iii) where it was unusual for the approach to the question to indicate the candidate had realised that the alkali was neutralising two different acids (HCl and \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}\)) and again in part (d) where it was rare for the response to outline a practical solution to the problem, though quite a number of candidates suggested that the pH would become constant, presumably not realising that the pH would be dominated by the HCl catalyst. Most students could however carry out the more routine tasks of writing an equilibrium constant expression and determining its value from the data given. Many candidates were aware of Le Chatelier effects on the position of equilibrium, but a significant number failed to use this information to answer the question actually asked and the unusual approach to the effect of temperature disconcerted many. Whilst most students managed to identify the ester as the component of the mixture that was insoluble in water, the reasons given were usually couched in terms of the polarity of the molecule (many quite polar molecules, halogenoalkanes for example, are insoluble in water) rather than its inability to form strong hydrogen bonds to water, which is the critical factor. Quite a number of students came up with a valid reason why water would not be a suitable solvent, though some students appeared to have overlooked the fact the question stated “other reason”.
Generally candidates found this question quite challenging and some left quite a number of parts unanswered. The tradition is that the first question on the paper is a data response question, which often addresses many aspects of the syllabus, and unfortunately candidates, especially those of average or below average ability, seem to have difficulty in tackling questions of this nature. One other issue with data response questions is that, of necessity, the data appears at the beginning of the question whilst, mainly because of the space left for candidates to answer, the later parts of the question referring to these data may not appear until a number of pages into the paper.
Part (a) concerning density, volume and amount of substance was generally reasonably well answered, but the following parts, concerning uncertainties, were rarely answered correctly and a number confused precision (uncertainty, either absolute or as a percentage) and accuracy (percentage error in the value obtained). Many candidates also seemed to lack experimental common sense, simply taking an average that included an initial titre that was much larger than the concordant second and third titres, rather than excluding it. This lack of experimental “know how” was also evident in responses to (c) (iii) where it was unusual for the approach to the question to indicate the candidate had realised that the alkali was neutralising two different acids (HCl and \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}\)) and again in part (d) where it was rare for the response to outline a practical solution to the problem, though quite a number of candidates suggested that the pH would become constant, presumably not realising that the pH would be dominated by the HCl catalyst. Most students could however carry out the more routine tasks of writing an equilibrium constant expression and determining its value from the data given. Many candidates were aware of Le Chatelier effects on the position of equilibrium, but a significant number failed to use this information to answer the question actually asked and the unusual approach to the effect of temperature disconcerted many. Whilst most students managed to identify the ester as the component of the mixture that was insoluble in water, the reasons given were usually couched in terms of the polarity of the molecule (many quite polar molecules, halogenoalkanes for example, are insoluble in water) rather than its inability to form strong hydrogen bonds to water, which is the critical factor. Quite a number of students came up with a valid reason why water would not be a suitable solvent, though some students appeared to have overlooked the fact the question stated “other reason”.
Generally candidates found this question quite challenging and some left quite a number of parts unanswered. The tradition is that the first question on the paper is a data response question, which often addresses many aspects of the syllabus, and unfortunately candidates, especially those of average or below average ability, seem to have difficulty in tackling questions of this nature. One other issue with data response questions is that, of necessity, the data appears at the beginning of the question whilst, mainly because of the space left for candidates to answer, the later parts of the question referring to these data may not appear until a number of pages into the paper.
Part (a) concerning density, volume and amount of substance was generally reasonably well answered, but the following parts, concerning uncertainties, were rarely answered correctly and a number confused precision (uncertainty, either absolute or as a percentage) and accuracy (percentage error in the value obtained). Many candidates also seemed to lack experimental common sense, simply taking an average that included an initial titre that was much larger than the concordant second and third titres, rather than excluding it. This lack of experimental “know how” was also evident in responses to (c) (iii) where it was unusual for the approach to the question to indicate the candidate had realised that the alkali was neutralising two different acids (HCl and \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}\)) and again in part (d) where it was rare for the response to outline a practical solution to the problem, though quite a number of candidates suggested that the pH would become constant, presumably not realising that the pH would be dominated by the HCl catalyst. Most students could however carry out the more routine tasks of writing an equilibrium constant expression and determining its value from the data given. Many candidates were aware of Le Chatelier effects on the position of equilibrium, but a significant number failed to use this information to answer the question actually asked and the unusual approach to the effect of temperature disconcerted many. Whilst most students managed to identify the ester as the component of the mixture that was insoluble in water, the reasons given were usually couched in terms of the polarity of the molecule (many quite polar molecules, halogenoalkanes for example, are insoluble in water) rather than its inability to form strong hydrogen bonds to water, which is the critical factor. Quite a number of students came up with a valid reason why water would not be a suitable solvent, though some students appeared to have overlooked the fact the question stated “other reason”.
Generally candidates found this question quite challenging and some left quite a number of parts unanswered. The tradition is that the first question on the paper is a data response question, which often addresses many aspects of the syllabus, and unfortunately candidates, especially those of average or below average ability, seem to have difficulty in tackling questions of this nature. One other issue with data response questions is that, of necessity, the data appears at the beginning of the question whilst, mainly because of the space left for candidates to answer, the later parts of the question referring to these data may not appear until a number of pages into the paper.
Part (a) concerning density, volume and amount of substance was generally reasonably well answered, but the following parts, concerning uncertainties, were rarely answered correctly and a number confused precision (uncertainty, either absolute or as a percentage) and accuracy (percentage error in the value obtained). Many candidates also seemed to lack experimental common sense, simply taking an average that included an initial titre that was much larger than the concordant second and third titres, rather than excluding it. This lack of experimental “know how” was also evident in responses to (c) (iii) where it was unusual for the approach to the question to indicate the candidate had realised that the alkali was neutralising two different acids (HCl and \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}\)) and again in part (d) where it was rare for the response to outline a practical solution to the problem, though quite a number of candidates suggested that the pH would become constant, presumably not realising that the pH would be dominated by the HCl catalyst. Most students could however carry out the more routine tasks of writing an equilibrium constant expression and determining its value from the data given. Many candidates were aware of Le Chatelier effects on the position of equilibrium, but a significant number failed to use this information to answer the question actually asked and the unusual approach to the effect of temperature disconcerted many. Whilst most students managed to identify the ester as the component of the mixture that was insoluble in water, the reasons given were usually couched in terms of the polarity of the molecule (many quite polar molecules, halogenoalkanes for example, are insoluble in water) rather than its inability to form strong hydrogen bonds to water, which is the critical factor. Quite a number of students came up with a valid reason why water would not be a suitable solvent, though some students appeared to have overlooked the fact the question stated “other reason”.
Generally candidates found this question quite challenging and some left quite a number of parts unanswered. The tradition is that the first question on the paper is a data response question, which often addresses many aspects of the syllabus, and unfortunately candidates, especially those of average or below average ability, seem to have difficulty in tackling questions of this nature. One other issue with data response questions is that, of necessity, the data appears at the beginning of the question whilst, mainly because of the space left for candidates to answer, the later parts of the question referring to these data may not appear until a number of pages into the paper.
Part (a) concerning density, volume and amount of substance was generally reasonably well answered, but the following parts, concerning uncertainties, were rarely answered correctly and a number confused precision (uncertainty, either absolute or as a percentage) and accuracy (percentage error in the value obtained). Many candidates also seemed to lack experimental common sense, simply taking an average that included an initial titre that was much larger than the concordant second and third titres, rather than excluding it. This lack of experimental “know how” was also evident in responses to (c) (iii) where it was unusual for the approach to the question to indicate the candidate had realised that the alkali was neutralising two different acids (HCl and \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}\)) and again in part (d) where it was rare for the response to outline a practical solution to the problem, though quite a number of candidates suggested that the pH would become constant, presumably not realising that the pH would be dominated by the HCl catalyst. Most students could however carry out the more routine tasks of writing an equilibrium constant expression and determining its value from the data given. Many candidates were aware of Le Chatelier effects on the position of equilibrium, but a significant number failed to use this information to answer the question actually asked and the unusual approach to the effect of temperature disconcerted many. Whilst most students managed to identify the ester as the component of the mixture that was insoluble in water, the reasons given were usually couched in terms of the polarity of the molecule (many quite polar molecules, halogenoalkanes for example, are insoluble in water) rather than its inability to form strong hydrogen bonds to water, which is the critical factor. Quite a number of students came up with a valid reason why water would not be a suitable solvent, though some students appeared to have overlooked the fact the question stated “other reason”.
Generally candidates found this question quite challenging and some left quite a number of parts unanswered. The tradition is that the first question on the paper is a data response question, which often addresses many aspects of the syllabus, and unfortunately candidates, especially those of average or below average ability, seem to have difficulty in tackling questions of this nature. One other issue with data response questions is that, of necessity, the data appears at the beginning of the question whilst, mainly because of the space left for candidates to answer, the later parts of the question referring to these data may not appear until a number of pages into the paper.
Part (a) concerning density, volume and amount of substance was generally reasonably well answered, but the following parts, concerning uncertainties, were rarely answered correctly and a number confused precision (uncertainty, either absolute or as a percentage) and accuracy (percentage error in the value obtained). Many candidates also seemed to lack experimental common sense, simply taking an average that included an initial titre that was much larger than the concordant second and third titres, rather than excluding it. This lack of experimental “know how” was also evident in responses to (c) (iii) where it was unusual for the approach to the question to indicate the candidate had realised that the alkali was neutralising two different acids (HCl and \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}\)) and again in part (d) where it was rare for the response to outline a practical solution to the problem, though quite a number of candidates suggested that the pH would become constant, presumably not realising that the pH would be dominated by the HCl catalyst. Most students could however carry out the more routine tasks of writing an equilibrium constant expression and determining its value from the data given. Many candidates were aware of Le Chatelier effects on the position of equilibrium, but a significant number failed to use this information to answer the question actually asked and the unusual approach to the effect of temperature disconcerted many. Whilst most students managed to identify the ester as the component of the mixture that was insoluble in water, the reasons given were usually couched in terms of the polarity of the molecule (many quite polar molecules, halogenoalkanes for example, are insoluble in water) rather than its inability to form strong hydrogen bonds to water, which is the critical factor. Quite a number of students came up with a valid reason why water would not be a suitable solvent, though some students appeared to have overlooked the fact the question stated “other reason”.
Generally candidates found this question quite challenging and some left quite a number of parts unanswered. The tradition is that the first question on the paper is a data response question, which often addresses many aspects of the syllabus, and unfortunately candidates, especially those of average or below average ability, seem to have difficulty in tackling questions of this nature. One other issue with data response questions is that, of necessity, the data appears at the beginning of the question whilst, mainly because of the space left for candidates to answer, the later parts of the question referring to these data may not appear until a number of pages into the paper.
Part (a) concerning density, volume and amount of substance was generally reasonably well answered, but the following parts, concerning uncertainties, were rarely answered correctly and a number confused precision (uncertainty, either absolute or as a percentage) and accuracy (percentage error in the value obtained). Many candidates also seemed to lack experimental common sense, simply taking an average that included an initial titre that was much larger than the concordant second and third titres, rather than excluding it. This lack of experimental “know how” was also evident in responses to (c) (iii) where it was unusual for the approach to the question to indicate the candidate had realised that the alkali was neutralising two different acids (HCl and \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}\)) and again in part (d) where it was rare for the response to outline a practical solution to the problem, though quite a number of candidates suggested that the pH would become constant, presumably not realising that the pH would be dominated by the HCl catalyst. Most students could however carry out the more routine tasks of writing an equilibrium constant expression and determining its value from the data given. Many candidates were aware of Le Chatelier effects on the position of equilibrium, but a significant number failed to use this information to answer the question actually asked and the unusual approach to the effect of temperature disconcerted many. Whilst most students managed to identify the ester as the component of the mixture that was insoluble in water, the reasons given were usually couched in terms of the polarity of the molecule (many quite polar molecules, halogenoalkanes for example, are insoluble in water) rather than its inability to form strong hydrogen bonds to water, which is the critical factor. Quite a number of students came up with a valid reason why water would not be a suitable solvent, though some students appeared to have overlooked the fact the question stated “other reason”.
Generally candidates found this question quite challenging and some left quite a number of parts unanswered. The tradition is that the first question on the paper is a data response question, which often addresses many aspects of the syllabus, and unfortunately candidates, especially those of average or below average ability, seem to have difficulty in tackling questions of this nature. One other issue with data response questions is that, of necessity, the data appears at the beginning of the question whilst, mainly because of the space left for candidates to answer, the later parts of the question referring to these data may not appear until a number of pages into the paper.
Part (a) concerning density, volume and amount of substance was generally reasonably well answered, but the following parts, concerning uncertainties, were rarely answered correctly and a number confused precision (uncertainty, either absolute or as a percentage) and accuracy (percentage error in the value obtained). Many candidates also seemed to lack experimental common sense, simply taking an average that included an initial titre that was much larger than the concordant second and third titres, rather than excluding it. This lack of experimental “know how” was also evident in responses to (c) (iii) where it was unusual for the approach to the question to indicate the candidate had realised that the alkali was neutralising two different acids (HCl and \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}\)) and again in part (d) where it was rare for the response to outline a practical solution to the problem, though quite a number of candidates suggested that the pH would become constant, presumably not realising that the pH would be dominated by the HCl catalyst. Most students could however carry out the more routine tasks of writing an equilibrium constant expression and determining its value from the data given. Many candidates were aware of Le Chatelier effects on the position of equilibrium, but a significant number failed to use this information to answer the question actually asked and the unusual approach to the effect of temperature disconcerted many. Whilst most students managed to identify the ester as the component of the mixture that was insoluble in water, the reasons given were usually couched in terms of the polarity of the molecule (many quite polar molecules, halogenoalkanes for example, are insoluble in water) rather than its inability to form strong hydrogen bonds to water, which is the critical factor. Quite a number of students came up with a valid reason why water would not be a suitable solvent, though some students appeared to have overlooked the fact the question stated “other reason”.
Generally candidates found this question quite challenging and some left quite a number of parts unanswered. The tradition is that the first question on the paper is a data response question, which often addresses many aspects of the syllabus, and unfortunately candidates, especially those of average or below average ability, seem to have difficulty in tackling questions of this nature. One other issue with data response questions is that, of necessity, the data appears at the beginning of the question whilst, mainly because of the space left for candidates to answer, the later parts of the question referring to these data may not appear until a number of pages into the paper.
Part (a) concerning density, volume and amount of substance was generally reasonably well answered, but the following parts, concerning uncertainties, were rarely answered correctly and a number confused precision (uncertainty, either absolute or as a percentage) and accuracy (percentage error in the value obtained). Many candidates also seemed to lack experimental common sense, simply taking an average that included an initial titre that was much larger than the concordant second and third titres, rather than excluding it. This lack of experimental “know how” was also evident in responses to (c) (iii) where it was unusual for the approach to the question to indicate the candidate had realised that the alkali was neutralising two different acids (HCl and \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}\)) and again in part (d) where it was rare for the response to outline a practical solution to the problem, though quite a number of candidates suggested that the pH would become constant, presumably not realising that the pH would be dominated by the HCl catalyst. Most students could however carry out the more routine tasks of writing an equilibrium constant expression and determining its value from the data given. Many candidates were aware of Le Chatelier effects on the position of equilibrium, but a significant number failed to use this information to answer the question actually asked and the unusual approach to the effect of temperature disconcerted many. Whilst most students managed to identify the ester as the component of the mixture that was insoluble in water, the reasons given were usually couched in terms of the polarity of the molecule (many quite polar molecules, halogenoalkanes for example, are insoluble in water) rather than its inability to form strong hydrogen bonds to water, which is the critical factor. Quite a number of students came up with a valid reason why water would not be a suitable solvent, though some students appeared to have overlooked the fact the question stated “other reason”.
The rate of reaction is an important factor in industrial processes such as the Contact process to make sulfur trioxide, \({\text{S}}{{\text{O}}_{\text{3}}}{\text{(g)}}\).
Define the term rate of reaction.
Describe the collision theory.
The Contact process involves this homogeneous equilibrium:
\[{\text{2S}}{{\text{O}}_{\text{2}}}{\text{(g)}} + {{\text{O}}_{\text{2}}}{\text{(g)}} \rightleftharpoons {\text{2S}}{{\text{O}}_{\text{3}}}{\text{(g)}}\,\,\,\,\,\Delta H = - 198{\text{ kJ}}\]
State and explain how increasing the pressure of the reaction mixture affects the yield of \({\text{S}}{{\text{O}}_{\text{3}}}\).
The Contact process involves this homogeneous equilibrium:
\[{\text{2S}}{{\text{O}}_{\text{2}}}{\text{(g)}} + {{\text{O}}_{\text{2}}}{\text{(g)}} \rightleftharpoons {\text{2S}}{{\text{O}}_{\text{3}}}{\text{(g)}}\,\,\,\,\,\Delta H = - 198{\text{ kJ}}\]
2.00 mol of \({\text{S}}{{\text{O}}_{\text{2}}}{\text{(g)}}\) are mixed with 3.00 mol of \({{\text{O}}_{\text{2}}}{\text{(g)}}\) in a \({\text{1.00 d}}{{\text{m}}^{\text{3}}}\) container until equilibrium is reached. At equilibrium there are 0.80 mol of \({\text{S}}{{\text{O}}_{\text{3}}}{\text{(g)}}\).
Determine the equilibrium constant (\({K_{\text{c}}}\)) assuming all gases are at the same temperature and pressure.
The Contact process involves this homogeneous equilibrium:
\[{\text{2S}}{{\text{O}}_{\text{2}}}{\text{(g)}} + {{\text{O}}_{\text{2}}}{\text{(g)}} \rightleftharpoons {\text{2S}}{{\text{O}}_{\text{3}}}{\text{(g)}}\,\,\,\,\,\Delta H = - 198{\text{ kJ}}\]
State the effect of increasing temperature on the value of \({K_{\text{c}}}\) for this reaction.
Outline the economic importance of using a catalyst in the Contact process.
Markscheme
change in concentration of reactant/product with time / rate of change of concentration;
Accept “increase” instead of “change” for product and “decrease” instead of “change” for reactant.
Accept “mass/amount/volume” instead of “concentration”.
Do not accept substance.
collision frequency;
two particles must collide;
particles must have sufficient energy to overcome the activation energy/\(E \geqslant {E_a}\);
Concept of activation energy must be mentioned.
appropriate collision geometry/orientation;
increases yield;
(equilibrium shifts to the right/products as) more gaseous moles in reactants/on left / fewer gaseous moles in products/on right;
\({\text{Eqm[}}{{\text{O}}_2}{\text{]}} = {\text{2.6 (mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{)}}\);
\({\text{Eqm[S}}{{\text{O}}_2}{\text{]}} = {\text{1.2 (mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{)}}\);
\({K_{\text{c}}} = \frac{{{{{\text{[S}}{{\text{O}}_3}]}^2}}}{{{{{\text{[S}}{{\text{O}}_2}{\text{]}}}^2}{\text{[}}{{\text{O}}_2}{\text{]}}}}\);
\({K_{\text{c}}} = 0.17\);
Award [4] for correct final answer.
Ignore units.
\({\text{(}}{K_{\text{c}}}{\text{)}}\) decreases;
catalyst increases rate of reaction / equilibrium reached faster / increases yield of product per unit time;
reduces costs / reduces energy needed;
Do not accept just “increases the yield”.
Examiners report
The definitions of rate of reaction in (a) were poor with many referring to a measure of time rather than a change in concentration. The collision theory was described successfully for the most part with “frequency of collisions” less frequently mentioned. In (c) (i) most realized that the number of moles of gases is important and thus gave a correct answer. Whilst the \({K_{\text{c}}}\) expression was often given correctly in (ii), the calculation of equilibrium mole concentrations was more testing, particularly that for \({\text{[}}{{\text{O}}_{\text{2}}}{\text{]}}\). Many were able to answer (iii) correctly. In part (d) many suggested that it is good to make more of something rather than relating this to a reduction in costs.
The definitions of rate of reaction in (a) were poor with many referring to a measure of time rather than a change in concentration. The collision theory was described successfully for the most part with “frequency of collisions” less frequently mentioned. In (c) (i) most realized that the number of moles of gases is important and thus gave a correct answer. Whilst the \({K_{\text{c}}}\) expression was often given correctly in (ii), the calculation of equilibrium mole concentrations was more testing, particularly that for \({\text{[}}{{\text{O}}_{\text{2}}}{\text{]}}\). Many were able to answer (iii) correctly. In part (d) many suggested that it is good to make more of something rather than relating this to a reduction in costs.
The definitions of rate of reaction in (a) were poor with many referring to a measure of time rather than a change in concentration. The collision theory was described successfully for the most part with “frequency of collisions” less frequently mentioned. In (c) (i) most realized that the number of moles of gases is important and thus gave a correct answer. Whilst the \({K_{\text{c}}}\) expression was often given correctly in (ii), the calculation of equilibrium mole concentrations was more testing, particularly that for \({\text{[}}{{\text{O}}_{\text{2}}}{\text{]}}\). Many were able to answer (iii) correctly. In part (d) many suggested that it is good to make more of something rather than relating this to a reduction in costs.
The definitions of rate of reaction in (a) were poor with many referring to a measure of time rather than a change in concentration. The collision theory was described successfully for the most part with “frequency of collisions” less frequently mentioned. In (c) (i) most realized that the number of moles of gases is important and thus gave a correct answer. Whilst the \({K_{\text{c}}}\) expression was often given correctly in (ii), the calculation of equilibrium mole concentrations was more testing, particularly that for \({\text{[}}{{\text{O}}_{\text{2}}}{\text{]}}\). Many were able to answer (iii) correctly. In part (d) many suggested that it is good to make more of something rather than relating this to a reduction in costs.
The definitions of rate of reaction in (a) were poor with many referring to a measure of time rather than a change in concentration. The collision theory was described successfully for the most part with “frequency of collisions” less frequently mentioned. In (c) (i) most realized that the number of moles of gases is important and thus gave a correct answer. Whilst the \({K_{\text{c}}}\) expression was often given correctly in (ii), the calculation of equilibrium mole concentrations was more testing, particularly that for \({\text{[}}{{\text{O}}_{\text{2}}}{\text{]}}\). Many were able to answer (iii) correctly. In part (d) many suggested that it is good to make more of something rather than relating this to a reduction in costs.
The definitions of rate of reaction in (a) were poor with many referring to a measure of time rather than a change in concentration. The collision theory was described successfully for the most part with “frequency of collisions” less frequently mentioned. In (c) (i) most realized that the number of moles of gases is important and thus gave a correct answer. Whilst the \({K_{\text{c}}}\) expression was often given correctly in (ii), the calculation of equilibrium mole concentrations was more testing, particularly that for \({\text{[}}{{\text{O}}_{\text{2}}}{\text{]}}\). Many were able to answer (iii) correctly. In part (d) many suggested that it is good to make more of something rather than relating this to a reduction in costs.
The rate of the acid-catalysed iodination of propanone can be followed by measuring how the concentration of iodine changes with time.
I2(aq) + CH3COCH3(aq) → CH3COCH2I(aq) + H+(aq) + I−(aq)
The general form of the rate equation is:
Rate = [H3CCOCH3(aq)]m × [I2(aq)]n × [H+(aq)]p
The reaction is first order with respect to propanone.
Suggest how the change of iodine concentration could be followed.
A student produced these results with \([{{\text{H}}^ + }] = 0.15{\text{ mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\). Propanone and acid were in excess and iodine was the limiting reagent. Determine the relative rate of reaction when \([{{\text{H}}^ + }] = 0.15{\text{ mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\).
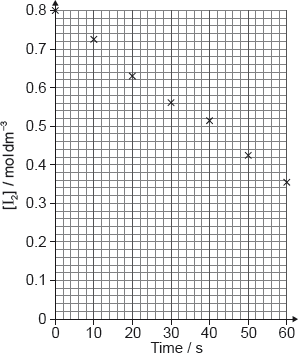
The student then carried out the experiment at other acid concentrations with all other conditions remaining unchanged.
Determine the relationship between the rate of reaction and the concentration of acid and the order of reaction with respect to hydrogen ions.
When the concentration of iodine is varied, while keeping the concentrations of acid and propanone constant, the following graphs are obtained.
Deduce, giving your reason, the order of reaction with respect to iodine.
When the reaction is carried out in the absence of acid the following graph is obtained.
Discuss the shape of the graph between A and B.
Markscheme
use a colorimeter/monitor the change in colour
OR
take samples AND quench AND titrate «with thiosulfate»
Accept change in pH.
Accept change in conductivity.
Accept other suitable methods.
Method must imply “change”.
[1 mark]
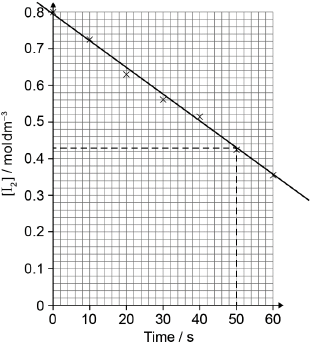
best fit line
relative rate of reaction \( = \ll \frac{{ - \Delta y}}{{\Delta x}} = \frac{{ - (0.43 - 0.80)}}{{50}} = \gg {\text{ }}0.0074/7.4 \times {10^{ - 3}}\)
Best fit line required for M1.
M2 is independent of M1.
Accept range from 0.0070 to 0.0080.
[2 marks]
Relationship:
rate of reaction is «directly» proportional to [H+]
OR
rate of reaction \(\alpha \) [H+]
Order of reaction with respect to [H+]:
first
Accept "doubling the concentration doubles the rate".
Do not accept “rate increases as concentration increases”.
[2 marks]
zero order
rate of reaction is the same for all concentrations of iodine
Accept “all graphs have same/similar gradient”.
[2 marks]
slow rate of reaction which gradually increases
as H+ ions are produced «to catalyse the reaction»
OR
reaction is autocatalytic
M1 should mention “rate of reaction”.
[2 marks]
Examiners report
The reaction between hydrogen and nitrogen monoxide is thought to proceed by the mechanism shown below.
(i) State the equation for the overall reaction.
(ii) Deduce the rate expression consistent with this mechanism.
(iii) Explain how you would attempt to confirm this rate expression, giving the results you would expect.
(iv) State, giving your reason, whether confirmation of the rate expression would prove that the mechanism given is correct.
(v) Suggest how the rate of this reaction could be measured experimentally.
The enthalpy change for the reaction between nitrogen monoxide and hydrogen is −664 kJ and its activation energy is 63 kJ.
(i) Sketch the potential energy profile for the overall reaction, using the axes given, indicating both the enthalpy of reaction and activation energy.
(ii) This reaction is normally carried out using a catalyst. Draw a dotted line labelled “Catalysed” on the diagram above to indicate the effect of the catalyst.
(iii) Sketch and label a second Maxwell–Boltzmann energy distribution curve representing the same system but at a higher temperature, Thigher.
(iv) Explain why an increase in temperature increases the rate of this reaction.
One of the intermediates in the reaction between nitrogen monoxide and hydrogen is dinitrogen monoxide, N2O. This can be represented by the resonance structures below:
(i) Analyse the bonding in dinitrogen monoxide in terms of σ-bonds and Δ-bonds.
(ii) State what is meant by resonance.
Markscheme
(i)
2NO(g) + 2H2(g) → N2(g) + 2H2O(g)
(ii)
rate = k [NO]2[H2]
(iii)
test the effect «on the reaction rate» of varying each concentration «independently»
OR
test the effect of varying [NO] «on rate», whilst keeping [H2] constant AND test effect of varying [H2] «on rate», whilst keeping [NO] constant
rate proportional to [NO]2
OR
doubling [NO] quadruples rate
rate proportional to [H2]
OR
doubling [H2] doubles rate
Remember to refer back to a (ii) for ECF.
If only one species in rate expression, third mark can be awarded for zero order discussion.
(iv)
no AND different mechanisms could give the same rate expression
OR
no AND mechanisms can only be disproved
OR
no AND just suggest it is consistent with the mechanism given
OR
no AND does not give information about what occurs after RDS
(v)
change of pressure «at constant volume and temperature» with time
OR
change of volume «at constant pressure and temperature» with time
Accept other methods where rate can be monitored with time
(i)
products lower than reactants AND enthalpy of reaction correctly marked and labelled with name or value
activation energy correctly marked and labelled with name or value
Accept other clear ways of indicating energy/ enthalpy changes.
(ii)
lower dotted curve, between same reactants and products levels, labelled “Catalysed”
(iii)
second curve at a higher temperature is correctly drawn (maximum lower and to right of original)
(iv)
greater proportion of molecules have E ≥ Ea or E > Ea
OR
greater area under curve to the right of the Ea
greater frequency of collisions «between molecules»
OR
more collisions per unit time/second
Do not accept just particles have greater kinetic energy.
Do not accept just “more collisions”.
(i)
ALTERNATIVE 1:
σ-bond from N to N AND from N to O
π-bond from N to N
delocalized π-bond/π-electrons «extending over the oxygen and both nitrogens»
ALTERNATIVE 2:
both have 2 σ-bonds «from N to N and from N to O» AND π-bond from N to N
one structure has second π-bond from N to N and the other has π-bond from N to O
delocalized π-bond/π-electrons
Award [1 max] if candidate has identified both/either structure having 2 σ-bonds and 2 π-bonds
(ii)
more than one possible position for a multiple/π-/pi- bond
Accept “more than one possible Lewis structure”.
Accept reference to delocalisation if M3 not awarded in c (i).
Accept reference to fractional bond orders.